A full-length glycoprotein mRNA vaccine confers complete protection against severe fever with thrombocytopenia syndrome virus, with broad-spectrum protective effects against bandaviruses
- PMID: 38829138
- PMCID: PMC11265342
- DOI: 10.1128/jvi.00769-24
A full-length glycoprotein mRNA vaccine confers complete protection against severe fever with thrombocytopenia syndrome virus, with broad-spectrum protective effects against bandaviruses
Abstract
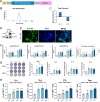
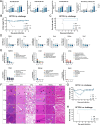
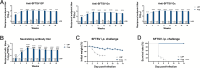
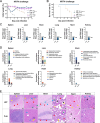
Highly pathogenic viruses from family Phenuiviridae, which are mainly transmitted by arthropods, have intermittently sparked epidemics worldwide. In particular, tick-borne bandaviruses, such as severe fever with thrombocytopenia syndrome virus (SFTSV), continue to spread in mountainous areas, resulting in an average mortality rate as high as 10.5%, highlighting the urgency and importance of vaccine development. Here, an mRNA vaccine developed based on the full-length SFTSV glycoprotein, containing both the receptor-binding domain and the fusion domain, was shown to confer complete protection against SFTSV at a very low dose by triggering a type 1 helper T cell-biased cellular immune response in rodents. Moreover, the vaccine candidate elicited long-term immunity and protection against SFTSV for at least 5 months. Notably, it provided complete cross-protection against other bandaviruses, such as the Heartland virus and Guertu virus, in lethal challenge models. Further research revealed that the conserved epitopes among bandaviruses within the full-length SFTSV glycoprotein may facilitate broad-spectrum protection mediated by the cellular immune response. Collectively, these findings demonstrate that the full-length SFTSV glycoprotein mRNA vaccine is a promising vaccine candidate for SFTSV and other bandaviruses, and provide guidance for the development of broad-spectrum vaccines from conserved antigens and epitopes.
Importance: Tick-borne bandaviruses, such as SFTSV and Heartland virus, sporadically trigger outbreaks in addition to influenza viruses and coronaviruses, yet there are no specific vaccines or therapeutics against them. mRNA vaccine technology has advantages in terms of enabling in situ expression and triggering cellular immunity, thus offering new solutions for vaccine development against intractable viruses, such as bandaviruses. In this study, we developed a novel vaccine candidate for SFTSV by employing mRNA vaccination technology and using a full-length glycoprotein as an antigen target. This candidate vaccine confers complete and durable protection against SFTSV at a notably low dose while also providing cross-protection against Heartland virus and Guertu virus. This study highlights the prospective value of full-length SFTSV-glycoprotein-based mRNA vaccines and suggests a potential strategy for broad-spectrum bandavirus vaccines.
Keywords: broad-spectrum protection; cellular immunology; conserved epitope; glycoprotein; severe fever with thrombocytopenia syndrome virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Latest advances and prospects in the pathogenesis, animal models, and vaccine research of severe fever with thrombocytopenia syndrome virus.Front Immunol. 2025 Jun 26;16:1624290. doi: 10.3389/fimmu.2025.1624290. eCollection 2025. Front Immunol. 2025. PMID: 40642074 Free PMC article. Review.
-
The immunogenicity and protection efficacy evaluation of mRNA vaccine candidate for severe fever with thrombocytopenia syndrome in mice.PLoS Negl Trop Dis. 2025 Apr 30;19(4):e0012999. doi: 10.1371/journal.pntd.0012999. eCollection 2025 Apr. PLoS Negl Trop Dis. 2025. PMID: 40305552 Free PMC article.
-
A rabies virus vectored severe fever with thrombocytopenia syndrome (SFTS) bivalent candidate vaccine confers protective immune responses in mice.Vet Microbiol. 2021 Jun;257:109076. doi: 10.1016/j.vetmic.2021.109076. Epub 2021 Apr 21. Vet Microbiol. 2021. PMID: 33957572
-
Development of a candidate vaccine against severe fever with thrombocytopenia syndrome virus using Gn/Gc glycoprotein via multiple expression vectors delivered by attenuated Salmonella confers effective protection in hDC-SIGN transduced mice.Vaccine. 2025 Jan 1;43(Pt 1):126524. doi: 10.1016/j.vaccine.2024.126524. Epub 2024 Nov 14. Vaccine. 2025. PMID: 39547019
-
Vaccine Development for Severe Fever with Thrombocytopenia Syndrome.Viruses. 2021 Apr 6;13(4):627. doi: 10.3390/v13040627. Viruses. 2021. PMID: 33917632 Free PMC article. Review.
Cited by
-
Safety, Immunogenicity, and Efficacy of a Recombinant Vesicular Stomatitis Virus Vectored Vaccine Against Severe Fever with Thrombocytopenia Syndrome Virus and Heartland Bandavirus.Vaccines (Basel). 2024 Dec 12;12(12):1403. doi: 10.3390/vaccines12121403. Vaccines (Basel). 2024. PMID: 39772063 Free PMC article.
-
Latest advances and prospects in the pathogenesis, animal models, and vaccine research of severe fever with thrombocytopenia syndrome virus.Front Immunol. 2025 Jun 26;16:1624290. doi: 10.3389/fimmu.2025.1624290. eCollection 2025. Front Immunol. 2025. PMID: 40642074 Free PMC article. Review.
-
mRNA Vaccine Development in the Fight Against Zoonotic Viral Diseases.Viruses. 2025 Jul 8;17(7):960. doi: 10.3390/v17070960. Viruses. 2025. PMID: 40733577 Free PMC article. Review.
-
Recent research advances in the development of Dabie Banda virus vaccines.PLoS Negl Trop Dis. 2024 Aug 29;18(8):e0012411. doi: 10.1371/journal.pntd.0012411. eCollection 2024 Aug. PLoS Negl Trop Dis. 2024. PMID: 39207951 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

