ATP-binding cassette family C member 1 constrains metabolic responses to high-fat diet in male mice
- PMID: 38829241
- PMCID: PMC11301423
- DOI: 10.1530/JOE-24-0024
ATP-binding cassette family C member 1 constrains metabolic responses to high-fat diet in male mice
Abstract
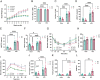
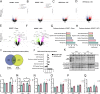
Glucocorticoids modulate glucose homeostasis, acting on metabolically active tissues such as liver, skeletal muscle, and adipose tissue. Intracellular regulation of glucocorticoid action in adipose tissue impacts metabolic responses to obesity. ATP-binding cassette family C member 1 (ABCC1) is a transmembrane glucocorticoid transporter known to limit the accumulation of exogenously administered corticosterone in adipose tissue. However, the role of ABCC1 in the regulation of endogenous glucocorticoid action and its impact on fuel metabolism has not been studied. Here, we investigate the impact of Abcc1 deficiency on glucocorticoid action and high-fat-diet (HFD)-induced obesity. In lean male mice, deficiency of Abcc1 increased endogenous corticosterone levels in skeletal muscle and adipose tissue but did not impact insulin sensitivity. In contrast, Abcc1-deficient male mice on HFD displayed impaired glucose and insulin tolerance, and fasting hyperinsulinaemia, without alterations in tissue corticosterone levels. Proteomics and bulk RNA sequencing revealed that Abcc1 deficiency amplified the transcriptional response to an obesogenic diet in adipose tissue but not in skeletal muscle. Moreover, Abcc1 deficiency impairs key signalling pathways related to glucose metabolism in both skeletal muscle and adipose tissue, in particular those related to OXPHOS machinery and Glut4. Together, our results highlight a role for ABCC1 in regulating glucose homeostasis, demonstrating diet-dependent effects that are not associated with altered tissue glucocorticoid concentrations.
Keywords: glucocorticoids; homeostasis; metabolism; obesity; steroids; transport.
Conflict of interest statement
All the authors declare no conflict of interest, financial or otherwise. Ruth Andrew is the Co-Editor-in-Chief of the
Figures


Similar articles
-
ABCC1 confers tissue-specific sensitivity to cortisol versus corticosterone: A rationale for safer glucocorticoid replacement therapy.Sci Transl Med. 2016 Aug 17;8(352):352ra109. doi: 10.1126/scitranslmed.aaf9074. Sci Transl Med. 2016. PMID: 27535620
-
Carbonyl reductase 1 amplifies glucocorticoid action in adipose tissue and impairs glucose tolerance in lean mice.Mol Metab. 2021 Jun;48:101225. doi: 10.1016/j.molmet.2021.101225. Epub 2021 Mar 27. Mol Metab. 2021. PMID: 33785425 Free PMC article.
-
ABCC1 modulates negative feedback control of the hypothalamic-pituitary-adrenal axis in vivo in humans.Metabolism. 2022 Mar;128:155118. doi: 10.1016/j.metabol.2021.155118. Epub 2022 Jan 4. Metabolism. 2022. PMID: 34990712 Free PMC article. Clinical Trial.
-
Rac1 muscle knockout exacerbates the detrimental effect of high-fat diet on insulin-stimulated muscle glucose uptake independently of Akt.J Physiol. 2018 Jun;596(12):2283-2299. doi: 10.1113/JP275602. Epub 2018 May 10. J Physiol. 2018. PMID: 29749029 Free PMC article.
-
Enhanced lipid oxidation and maintenance of muscle insulin sensitivity despite glucose intolerance in a diet-induced obesity mouse model.PLoS One. 2013 Aug 12;8(8):e71747. doi: 10.1371/journal.pone.0071747. eCollection 2013. PLoS One. 2013. PMID: 23951235 Free PMC article.
References
-
- Agudelo LZ, Ferreira DMS, Cervenka I, Bryzgalova G, Dadvar S, Jannig PR, Pettersson-Klein AT, Lakshmikanth T, Sustarsic EG, Porsmyr-Palmertz M, et al.2018Kynurenic acid and Gpr35 regulate adipose tissue energy homeostasis and inflammation. Cell Metabolism 27378–392.e5. (10.1016/j.cmet.2018.01.004) - DOI - PubMed
-
- Anderson AJ Andrew R Homer NZ Jones GC Smith K Livingstone DE Walker BR & Stimson RH. 2016Metformin increases cortisol regeneration by 11betaHSD1 in obese men with and without type 2 diabetes mellitus. Journal of Clinical Endocrinology and Metabolism 1013787–3793. (10.1210/jc.2016-2069) - DOI - PMC - PubMed
-
- Bankier S Wang L Crawford A Morgan RA Ruusalepp A Andrew R Bjorkegren JLM Walker BR & Michoel T. 2023Plasma cortisol-linked gene networks in hepatic and adipose tissues implicate corticosteroid-binding globulin in modulating tissue glucocorticoid action and cardiovascular risk. Frontiers in Endocrinology 141186252. (10.3389/fendo.2023.1186252) - DOI - PMC - PubMed
-
- Bell RMB, Villalobos E, Nixon M, Miguelez-Crespo A, Murphy L, Fawkes A, Coutts A, Sharp MGF, Koerner MV, Allan E, et al.2021Carbonyl reductase 1 amplifies glucocorticoid action in adipose tissue and impairs glucose tolerance in lean mice. Molecular Metabolism 48101225. (10.1016/j.molmet.2021.101225) - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

