Analysis of Legacy and Novel Neutral Per- and Polyfluoroalkyl Substances in Soils from an Industrial Manufacturing Facility
- PMID: 38829283
- PMCID: PMC11304343
- DOI: 10.1021/acs.est.3c10268
Analysis of Legacy and Novel Neutral Per- and Polyfluoroalkyl Substances in Soils from an Industrial Manufacturing Facility
Abstract
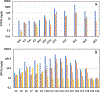
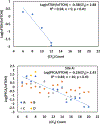
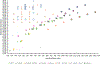
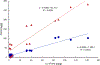
Per- and polyfluoroalkyl substances (PFASs) have been detected in an array of environmental media due to their ubiquitous use in industrial and consumer products as well as potential release from fluorochemical manufacturing facilities. During their manufacture, many fluorotelomer (FT) facilities rely on neutral intermediates in polymer production including the FT-alcohols (FTOHs). These PFAS are known to transform to the terminal acids (perfluoro carboxylic acids; PFCAs) at rates that vary with environmental conditions. In the current study on soils from a FT facility, we employed gas chromatography coupled with conventional- and high-resolution mass spectrometry (GC-MS and GC-HRMS) to investigate the profile of these precursor compounds, the intermediary secondary alcohols (sFTOHs), FT-acrylates (FTAcr), and FT-acetates (FTAce) in soils around the former FT-production facility. Of these precursors, the general trend in detection intensity was [FTOHs] > [sFTOHs] > [FTAcrs], while for the FTOHs, homologue intensities generally were [12:2 FTOH] > [14:2 FTOH] > [16:2 FTOH] > [10:2 FTOH] > [18:2 FTOH] > [20:2 FTOH] > [8:2 FTOH] ∼ [6:2 FTOH]. The corresponding terminal acids were also detected in all soil samples and positively correlated with the precursor concentrations. GC-HRMS confirmed the presence of industrial manufacturing byproducts such as FT-ethers and FT-esters and aided in the tentative identification of previously unreported dimers and other compounds. The application of GC-HRMS to the measurement and identification of precursor PFAS is in its infancy, but the methodologies described here will help refine its use in tentatively identifying these compounds in the environment.
Keywords: PFCA precursors; Semivolatile PFAS; contaminated soils; fluorotelomer alcohols; neutral PFAS.
Figures
References
-
- Buck RC; Franklin J; Berger U; Conder JM; Cousins IT; de Voogt P; Jensen AA; Kannan K; Mabury SA; van Leeuwen SP Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integrated Environ. Assess. Manag 2011, 7 (4), 513–541, DOI: 10.1002/ieam.258 - DOI - PMC - PubMed
-
- Kissa E. Fluorinated Surfactants and Repellents; Marcel Dekker AG, 2001;.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

