Real-World Evidence on Levetiracetam-Induced Hypokalemia: An Active Comparator Cohort Study
- PMID: 38829496
- PMCID: PMC11176145
- DOI: 10.1007/s40801-024-00431-4
Real-World Evidence on Levetiracetam-Induced Hypokalemia: An Active Comparator Cohort Study
Abstract
Background: Levetiracetam is an anti-seizure medication (ASM) with an established safety profile. However, a potential safety signal of hypokalemia following levetiracetam use was published in the World Health Organization newsletter.
Objective: To investigate the possible causal association between the use of levetiracetam and the development of hypokalemia.
Method: This was a new-user, active-comparator retrospective cohort study using Real-world Evidence Research Network data at the Saudi Food and Drug Authority from 2016 to 2022. Adults (≥ 18 years old) with an incident prescription for either levetiracetam or carbamazepine were followed for up to 6 months from the prescription date. Hypokalemia was ascertained by using diagnostic code (i.e., E87.6) or by serum potassium level below 3.5 mmol/L. A Cox proportional hazards model, adjusted with stabilized inverse probability of treatment weight, was fitted to compare the hazard of hypokalemia between levetiracetam and carbamazepine exposed patients.
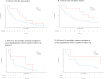
Results: A total of 8,982 patients entered the study cohort. The incidence rate of hypokalemia was 303 cases per 10,000 patient-years in the levetiracetam-exposed cohort compared to 57 cases per 10,000 patient-years among carbamazepine users. Compared to carbamazepine users, patients exposed to levetiracetam had an adjusted hazard ratio related to induced hypokalemia of 1.99 (95% confidence interval, 0.88-4.49). Results of sensitivity analyses were comparable to the main analysis.
Conclusion: The hazard ratio for hypokalemia with the use of levetiracetam versus carbamazepine was statistically comparable. However, the potential association between levetiracetam use and hypokalemia cannot be ruled out given the elevated hazard ratios from the main and sensitivity analyses. Further studies may provide a more precise assessment of this association.
© 2024. The Author(s).
Conflict of interest statement
Ohoud Almadani, Raseel Alrobe, Almaha Alfakhri, Sumaya Almohareb, Turki Althunian, and Adel Alrwisan declare that they have no potential conflicts of interest that might be relevant to the contents of this article.
Figures
Similar articles
-
A Rare Case of Levetiracetam-Induced Refractory Hypokalemia.Cureus. 2022 Apr 4;14(4):e23817. doi: 10.7759/cureus.23817. eCollection 2022 Apr. Cureus. 2022. PMID: 35530900 Free PMC article.
-
Levetiracetam as a first-line antiseizure medication in WHO grade 2 glioma: Time to seizure freedom and rates of treatment failure.Epilepsia. 2023 Apr;64(4):857-865. doi: 10.1111/epi.17508. Epub 2023 Jan 23. Epilepsia. 2023. PMID: 36636895
-
Association Between Antiseizure Drug Monotherapy and Mortality for Patients With Poststroke Epilepsy.JAMA Neurol. 2022 Feb 1;79(2):169-175. doi: 10.1001/jamaneurol.2021.4584. JAMA Neurol. 2022. PMID: 34902006 Free PMC article.
-
Carbamazepine versus phenytoin monotherapy for epilepsy: an individual participant data review.Cochrane Database Syst Rev. 2019 Jul 18;7(7):CD001911. doi: 10.1002/14651858.CD001911.pub4. Cochrane Database Syst Rev. 2019. PMID: 31318037 Free PMC article.
-
Levetiracetam for Seizure Prophylaxis in Neurocritical Care: A Systematic Review and Meta-analysis.Neurocrit Care. 2022 Feb;36(1):248-258. doi: 10.1007/s12028-021-01296-z. Epub 2021 Jul 20. Neurocrit Care. 2022. PMID: 34286461
References
-
- Kim M, Valerio C, Knobloch G. Potassium Disorders: Hypokalemia and Hyperkalemia. Am Fam Physician. 2023;107(1):59–70. - PubMed
LinkOut - more resources
Full Text Sources

