Patient-Reported Outcomes in Phase 3 Clinical Trials for Blood Cancers: A Systematic Review
- PMID: 38829615
- PMCID: PMC11148691
- DOI: 10.1001/jamanetworkopen.2024.14425
Patient-Reported Outcomes in Phase 3 Clinical Trials for Blood Cancers: A Systematic Review
Abstract
Importance: Published research suggests that patient-reported outcomes (PROs) are neither commonly collected nor reported in randomized clinical trials (RCTs) for solid tumors. Little is known about these practices in RCTs for hematological malignant neoplasms.
Objective: To evaluate the prevalence of PROs as prespecified end points in RCTs of hematological malignant neoplasms, and to assess reporting of PROs in associated trial publications.
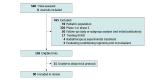
Evidence review: All issues of 8 journals known for publishing high-impact RCTs (NEJM, Lancet, Lancet Hematology, Lancet Oncology, Journal of Clinical Oncology, Blood, JAMA, and JAMA Oncology) between January 1, 2018, and December 13, 2022, were searched for primary publications of therapeutic phase 3 trials for adults with hematological malignant neoplasms. Studies that evaluated pretransplant conditioning regimens, graft-vs-host disease treatment, or radiotherapy as experimental treatment were excluded. Data regarding trial characteristics and PROs were extracted from manuscripts and trial protocols. Univariable analyses assessed associations between trial characteristics and PRO collection or reporting.
Findings: Ninety RCTs were eligible for analysis. PROs were an end point in 66 (73%) trials: in 1 trial (1%) as a primary end point, in 50 (56%) as a secondary end point, and in 15 (17%) as an exploratory end point. PRO data were reported in 26 of 66 primary publications (39%): outcomes were unchanged in 18 and improved in 8, with none reporting worse PROs with experimental treatment. Trials sponsored by for-profit entities were more likely to include PROs as an end point (49 of 55 [89%] vs 17 of 35 [49%]; P < .001) but were not significantly more likely to report PRO data (20 of 49 [41%] vs 6 of 17 [35%]; P = .69). Compared with trials involving lymphoma (18 of 29 [62%]) or leukemia or myelodysplastic syndrome (18 of 28 [64%]), those involving plasma cell disorders or multiple myeloma (27 of 30 [90%]) or myeloproliferative neoplasms (3 of 3 [100%]) were more likely to include PROs as an end point (P = .03). Similarly, compared with trials involving lymphoma (3 of 18 [17%]) or leukemia or myelodysplastic syndrome (5 of 18 [28%]), those involving plasma cell disorders or multiple myeloma (16 of 27 [59%]) or myeloproliferative neoplasms (2 of 3 [67%]) were more likely to report PROs in the primary publication (P = .01).
Conclusions and relevance: In this systematic review, almost 3 of every 4 therapeutic RCTs for blood cancers collected PRO data; however, only 1 RCT included PROs as a primary end point. Moreover, most did not report resulting PRO data in the primary publication and when reported, PROs were either better or unchanged, raising concern for publication bias. This analysis suggests a critical gap in dissemination of data on the lived experiences of patients enrolled in RCTs for hematological malignant neoplasms.
Conflict of interest statement
Figures
Similar articles
-
Use and Reporting of Patient-Reported Outcomes in Trials of Palliative Radiotherapy: A Systematic Review.JAMA Netw Open. 2022 Sep 1;5(9):e2231930. doi: 10.1001/jamanetworkopen.2022.31930. JAMA Netw Open. 2022. PMID: 36136335 Free PMC article.
-
Association of Industry and Academic Sponsorship With Negative Phase 3 Oncology Trials and Reported Outcomes on Participant Survival: A Pooled Analysis.JAMA Netw Open. 2019 May 3;2(5):e193684. doi: 10.1001/jamanetworkopen.2019.3684. JAMA Netw Open. 2019. PMID: 31074821 Free PMC article. Review.
-
Deficiencies in health-related quality-of-life assessment and reporting: a systematic review of oncology randomized phase III trials published between 2012 and 2016.Ann Oncol. 2018 Dec 1;29(12):2288-2295. doi: 10.1093/annonc/mdy449. Ann Oncol. 2018. PMID: 30304498
-
Underreporting of patient-reported outcomes in cystic fibrosis randomized controlled trials using CONSORT-PRO and RoB 2.0.Respir Med Res. 2023 Jun;83:100962. doi: 10.1016/j.resmer.2022.100962. Epub 2022 Oct 9. Respir Med Res. 2023. PMID: 36563550
-
Prevalence of Multiplicity and Appropriate Adjustments Among Cardiovascular Randomized Clinical Trials Published in Major Medical Journals.JAMA Netw Open. 2020 Apr 1;3(4):e203082. doi: 10.1001/jamanetworkopen.2020.3082. JAMA Netw Open. 2020. PMID: 32301992 Free PMC article.
Cited by
-
Patient-Reported Outcome Measures (PROMS) in Lymphoma.Curr Oncol. 2025 May 1;32(5):265. doi: 10.3390/curroncol32050265. Curr Oncol. 2025. PMID: 40422524 Free PMC article.
-
Follicular lymphoma: contemporary clinical management with a focus on recent therapeutic advances.Korean J Intern Med. 2025 May;40(3):371-393. doi: 10.3904/kjim.2024.279. Epub 2025 Feb 21. Korean J Intern Med. 2025. PMID: 39987895 Free PMC article. Review.
-
Patient-reported outcomes in Philadelphia chromosome-positive acute lymphoblastic leukemia patients treated with ponatinib or imatinib: results from the PhALLCON trial.Leukemia. 2025 Jun;39(6):1342-1350. doi: 10.1038/s41375-025-02608-4. Epub 2025 Apr 16. Leukemia. 2025. PMID: 40240572 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous