Fosfenopril Attenuates Inflammatory Response in Diabetic Dry Eye Models by Inhibiting the TLR4/NF-κB/NLRP3 Signaling Pathway
- PMID: 38829670
- PMCID: PMC11156208
- DOI: 10.1167/iovs.65.6.2
Fosfenopril Attenuates Inflammatory Response in Diabetic Dry Eye Models by Inhibiting the TLR4/NF-κB/NLRP3 Signaling Pathway
Abstract
Purpose: The purpose of this study was to investigate the involvement of the TLR4/NF-κB/NLRP3 signaling pathway and its underlying mechanism in diabetic dry eye.
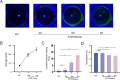
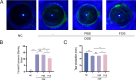
Methods: Two models of diabetic dry eye were established in high glucose-induced human corneal epithelial (HCE-T) cells and streptozotocin (STZ)-induced C57BL/6 mice, and the TLR4 inhibitor fosfenopril (FOS) was utilized to suppress the TLR4/NF-κB/NLRP3 signaling pathway. The expression changes in TLR4, NF-κB, NLRP3, and IL-1β, and other factors were detected by Western blot and RT‒qPCR, the wound healing rate was evaluated by cell scratch assay, and the symptoms of diabetic mice were evaluated by corneal sodium fluorescein staining and tear secretion assay.
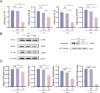
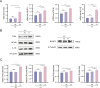
Results: In the diabetic dry eye model, the transcript levels of TLR4, NF-κB, NLRP3, and IL-1β were raised, and further application of FOS, a TLR4 inhibitor, downregulated the levels of these pathway factors. In addition, FOS was found to be effective in increasing the wound healing rate of high glucose-induced HCE-T cells, increasing tear production, and decreasing corneal fluorescence staining scores in diabetic mice, as measured by cell scratch assay, corneal sodium fluorescein staining assay, and tear production.
Conclusions: The current study found that the TLR4/NF-κB/NLRP3 signaling pathway regulates diabetic dry eye in an in vitro and in vivo model, and that FOS reduces the signs of dry eye in diabetic mice, providing a new treatment option for diabetic dry eye.
Conflict of interest statement
Disclosure:
Figures




Similar articles
-
Baicalin ameliorates inflammation response in diabetic dry eye models through the inhibition of the PERK/TXNIP/NLRP3 pathway.Exp Eye Res. 2025 Sep;258:110487. doi: 10.1016/j.exer.2025.110487. Epub 2025 Jun 13. Exp Eye Res. 2025. PMID: 40517884
-
Oridonin ameliorates ocular surface inflammatory responses by inhibiting the NLRP3/caspase-1/GSDMD pyroptosis pathway in dry eye.Exp Eye Res. 2024 Aug;245:109955. doi: 10.1016/j.exer.2024.109955. Epub 2024 Jun 4. Exp Eye Res. 2024. PMID: 38843984
-
Inhibition of HMGB1 improves necrotizing enterocolitis by inhibiting NLRP3 via TLR4 and NF-κB signaling pathways.J Cell Physiol. 2019 Aug;234(8):13431-13438. doi: 10.1002/jcp.28022. Epub 2019 Jan 7. J Cell Physiol. 2019. PMID: 30618088
-
Phytoconstituents as modulators of NF-κB signalling: Investigating therapeutic potential for diabetic wound healing.Biomed Pharmacother. 2024 Aug;177:117058. doi: 10.1016/j.biopha.2024.117058. Epub 2024 Jul 4. Biomed Pharmacother. 2024. PMID: 38968797 Review.
-
Correlation between gout and dry eye disease.Int Ophthalmol. 2024 Feb 20;44(1):102. doi: 10.1007/s10792-024-02965-6. Int Ophthalmol. 2024. PMID: 38376774 Free PMC article. Review.
Cited by
-
Single-Cell RNA Sequencing Revealed Functional Conjunctival Keratinocytes Loss via TGF-β-Wnt/β-Catenin Signaling in Sjögren's Syndrome Related Dry Eye.Invest Ophthalmol Vis Sci. 2025 Apr 1;66(4):43. doi: 10.1167/iovs.66.4.43. Invest Ophthalmol Vis Sci. 2025. PMID: 40238113 Free PMC article.
-
Role of Chinese Medicine Monomers in Dry Eye Disease: Breaking the Vicious Cycle of Inflammation.Pharmacol Res Perspect. 2025 Apr;13(2):e70077. doi: 10.1002/prp2.70077. Pharmacol Res Perspect. 2025. PMID: 39979080 Free PMC article. Review.
References
-
- Cho NH, Shaw JE, Karuranga S, et al. .. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pr. 2018; 138: 271–281. - PubMed
-
- Stapleton F, Alves M, Bunya VY, et al. .. TFOS DEWS II epidemiology report. Ocul Surf. 2017; 15: 334–365. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

