VHH Nanobody Versatility against Pentameric Ligand-Gated Ion Channels
- PMID: 38829690
- PMCID: PMC11181324
- DOI: 10.1021/acs.jmedchem.4c00231
VHH Nanobody Versatility against Pentameric Ligand-Gated Ion Channels
Erratum in
-
Corrections to "VHH Nanobody Versatility against Pentameric Ligand-Gated Ion Channels".J Med Chem. 2025 Jun 12;68(11):12286. doi: 10.1021/acs.jmedchem.4c02703. Epub 2025 Jun 2. J Med Chem. 2025. PMID: 40457707 Free PMC article. No abstract available.
Abstract
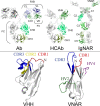
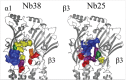
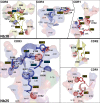
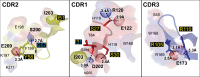
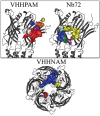
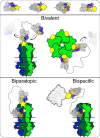
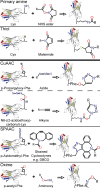
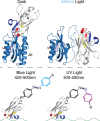
Pentameric ligand-gated ion channels provide rapid chemical-electrical signal transmission between cells in the central and peripheral nervous system. Their dysfunction is associated with many nervous system disorders. They are composed of five identical (homomeric receptors) or homologous (heteromeric receptors) subunits. VHH nanobodies, or single-chain antibodies, are the variable domain, VHH, of antibodies that are composed of the heavy chain only from camelids. Their unique structure results in many specific biochemical and biophysical properties that make them an excellent alternative to conventional antibodies. This Perspective explores the published VHH nanobodies which have been isolated against pentameric ligand-gated ion channel subfamilies. It outlines the genetic and chemical modifications available to alter nanobody function. An assessment of the available functional and structural data indicate that it is feasible to create therapeutic agents and impart, through their modification, a given desired modulatory effect of its target receptor for current stoichiometric-specific VHH nanobodies.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






Similar articles
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Optimization of a sarbecovirus llama nanobody-antigen binding interface via a combined computational and phage display protein engineering approach.Proc Natl Acad Sci U S A. 2025 Jul 15;122(28):e2426438122. doi: 10.1073/pnas.2426438122. Epub 2025 Jul 8. Proc Natl Acad Sci U S A. 2025. PMID: 40627384
-
Vestibular modulation by stimulant derivatives in a pentameric ligand-gated ion channel.Br J Pharmacol. 2025 Jun;182(12):2790-2802. doi: 10.1111/bph.70011. Epub 2025 Mar 11. Br J Pharmacol. 2025. PMID: 40065647
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
[Volume and health outcomes: evidence from systematic reviews and from evaluation of Italian hospital data].Epidemiol Prev. 2013 Mar-Jun;37(2-3 Suppl 2):1-100. Epidemiol Prev. 2013. PMID: 23851286 Italian.
References
-
- Hu H.; Ataka K.; Menny A.; Fourati Z.; Sauguet L.; Corringer P.-J.; Koehl P.; Heberle J.; Delarue M. Electrostatics, Proton Sensor, and Networks Governing the Gating Transition in GLIC, a Proton-Gated Pentameric Ion Channel. Proc. Natl. Acad. Sci. U. S. A. 2018, 115 (52), E12172–E12181. 10.1073/pnas.1813378116. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

