3D-printed porous zinc scaffold combined with bioactive serum exosomes promotes bone defect repair in rabbit radius
- PMID: 38829771
- PMCID: PMC11210218
- DOI: 10.18632/aging.205891
3D-printed porous zinc scaffold combined with bioactive serum exosomes promotes bone defect repair in rabbit radius
Abstract
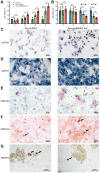
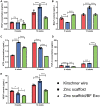
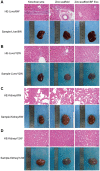
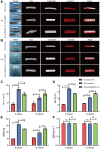
Currently, the repair of large bone defects still faces numerous challenges, with the most crucial being the lack of large bone grafts with good osteogenic properties. In this study, a novel bone repair implant (degradable porous zinc scaffold/BF Exo composite implant) was developed by utilizing laser melting rapid prototyping 3D printing technology to fabricate a porous zinc scaffold, combining it under vacuum conditions with highly bioactive serum exosomes (BF EXO) and Poloxamer 407 thermosensitive hydrogel. The electron microscope revealed the presence of tea saucer-shaped exosomes with a double-layered membrane structure, ranging in diameter from 30-150 nm, with an average size of 86.3 nm and a concentration of 3.28E+09 particles/mL. In vitro experiments demonstrated that the zinc scaffold displayed no significant cytotoxicity, and loading exosomes enhanced the zinc scaffold's ability to promote osteogenic cell activity while inhibiting osteoclast activity. In vivo experiments on rabbits indicated that the hepatic and renal toxicity of the zinc scaffold decreased over time, and the loading of exosomes alleviated the hepatic and renal toxic effects of the zinc scaffold. Throughout various stages of repairing radial bone defects in rabbits, loading exosomes reinforced the zinc scaffold's capacity to enhance osteogenic cell activity, suppress osteoclast activity, and promote angiogenesis. This effect may be attributed to BF Exo's regulation of p38/STAT1 signaling. This study signifies that the combined treatment of degradable porous zinc scaffolds and BF Exo is an effective and biocompatible strategy for bone defect repair therapy.
Keywords: 3D printing; bone defect; bone repair; exosome; zinc scaffold.
Conflict of interest statement
Figures





Similar articles
-
Degradable calcium phosphate-coated porous zinc scaffold combined with platelet-rich plasma implantation for rabbit radius defect.Eur J Med Res. 2025 May 31;30(1):437. doi: 10.1186/s40001-025-02676-3. Eur J Med Res. 2025. PMID: 40450358 Free PMC article.
-
Choline Phosphate Surface-Activated 3D-Printed Porous Titanium Scaffold Combined with Stem Cell Exosomes for Enhancing Bone Defects Repair.ACS Appl Mater Interfaces. 2025 May 14;17(19):27788-27805. doi: 10.1021/acsami.5c00953. Epub 2025 Apr 30. ACS Appl Mater Interfaces. 2025. PMID: 40304439
-
Engineering 3D-Printed Strontium-Titanium Scaffold-Integrated Highly Bioactive Serum Exosomes for Critical Bone Defects by Osteogenesis and Angiogenesis.ACS Appl Mater Interfaces. 2023 Jun 14;15(23):27486-27501. doi: 10.1021/acsami.3c00898. Epub 2023 May 22. ACS Appl Mater Interfaces. 2023. PMID: 37212747
-
Three-dimensional (3D) printed scaffold and material selection for bone repair.Acta Biomater. 2019 Jan 15;84:16-33. doi: 10.1016/j.actbio.2018.11.039. Epub 2018 Nov 24. Acta Biomater. 2019. PMID: 30481607 Review.
-
3D-printed porous tantalum artificial bone scaffolds: fabrication, properties, and applications.Biomed Mater. 2024 May 15;19(4). doi: 10.1088/1748-605X/ad46d2. Biomed Mater. 2024. PMID: 38697199 Review.
Cited by
-
Degradable calcium phosphate-coated porous zinc scaffold combined with platelet-rich plasma implantation for rabbit radius defect.Eur J Med Res. 2025 May 31;30(1):437. doi: 10.1186/s40001-025-02676-3. Eur J Med Res. 2025. PMID: 40450358 Free PMC article.
References
-
- Leu Alexa R, Cucuruz A, Ghiţulică CD, Voicu G, Stamat Balahura LR, Dinescu S, Vlasceanu GM, Iovu H, Serafim A, Ianchis R, Ciocan LT, Costache M. 3D Printed Composite Scaffolds of GelMA and Hydroxyapatite Nanopowders Doped with Mg/Zn Ions to Evaluate the Expression of Genes and Proteins of Osteogenic Markers. Nanomaterials (Basel). 2022; 12:3420. 10.3390/nano12193420 - DOI - PMC - PubMed
-
- Li C, Sun F, Tian J, Li J, Sun H, Zhang Y, Guo S, Lin Y, Sun X, Zhao Y. Continuously released Zn2+ in 3D-printed PLGA/β-TCP/Zn scaffolds for bone defect repair by improving osteoinductive and anti-inflammatory properties. Bioact Mater. 2022; 24:361–75. 10.1016/j.bioactmat.2022.12.015 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

