Functional trajectories before and after loss of ambulation in Duchenne muscular dystrophy and implications for clinical trials
- PMID: 38829874
- PMCID: PMC11146704
- DOI: 10.1371/journal.pone.0304099
Functional trajectories before and after loss of ambulation in Duchenne muscular dystrophy and implications for clinical trials
Abstract
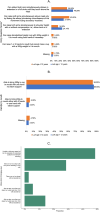
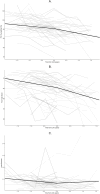
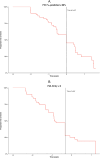
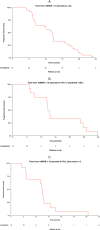
This study examined functional trajectories of subjects during the transition phase between ambulatory and non-ambulatory Duchenne muscular dystrophy (DMD) to inform clinical trial designs for new therapeutics. Ambulatory, pulmonary, and upper limb function leading up to loss of ambulation (LoA) and non-ambulatory measures following LoA were quantified; time ordering of pulmonary and upper limb milestones relative to LoA were determined; and the 10-second time threshold for 10-meter walk/run (10MWR) as a marker of approaching LOA was explored. Included in this analysis were 51 subjects aged between 7 and 18 years who experienced LoA during follow-up in the PRO-DMD-01 natural history study. Mean age at LoA was 12.7 (7.1-18.6) years. Mean annual rates of decline in forced vital capacity (FVC) <80%-predicted and performance of upper limb (PUL) 1.2 total score were smaller before than after LoA, but not significantly (FVC %-predicted: 5.6% vs. 10.1%, p = 0.21; PUL 1.2 total score: 2.3 vs. 3.8 units, p = 0.20). More than half of patients experienced clinically significant deficits in FVC %-predicted and PUL 1.2 before experiencing LoA. Among subjects with baseline 10MWR >10 s, those with <1 year to LoA had similar mean ages but significantly worse mean ambulatory function at baseline compared to those with ≥1 year to LoA. Enriching DMD clinical trials for patients with declining pulmonary or upper limb function is achievable without restricting enrollment to non-ambulatory patients. The sequencing of LoA and initial deficits in pulmonary and upper limb function varied across patients and highlights the potential for composite outcomes or multi-outcome trial designs to assess disease-modifying therapies more comprehensively.
Copyright: © 2024 McDonald et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
Craig McDonald has served as a consultant for PTC Therapeutics, BioMarin Pharmaceutical, Sarepta Therapeutics, Eli Lilly, Pfizer Inc, Santhera Pharmaceuticals, Cardero Therapeutics, Inc, Catabasis Pharmaceuticals, Capricor Therapeutics, Astellas Pharma (Mitobridge), and FibroGen, Inc; serves on external advisory boards related to DMD for PTC Therapeutics, Sarepta Therapeutics, Santhera Pharmaceuticals, and Capricor Therapeutics; and reports grants from US Department of Education/National Institute on Disability and Rehabilitation Research, the National Institute on Disability, Independent Living, and Rehabilitation Research, US NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH/National Institute of Neurologic Disorders and Stroke, US Department of Defense, and Parent Project Muscular Dystrophy US. James Signorovitch co-founded the collaborative Trajectory Analysis Project (cTAP) and is an employee of Analysis Group, Inc., a consulting firm that received funding from the sponsors of cTAP to conduct this study. Eugenio Mercuri has served on clinical steering committees and/or as a consultant for Eli Lilly, Italfarmaco, PTC Therapeutics, Sarepta, Santhera, and Pfizer; has served as PI for GlaxoSmithKline, Prosensa, BioMarin Pharmaceutical, Italfarmaco, Roche, PTC, Pfizer, Sarepta, Santhera, Wave, NS and Eli Lilly. Erik H. Niks is a member of the European Reference Network for Rare Neuromuscular Diseases (ERN EURO‐NMD). Erik H. Niks report grants from Spieren voor Spieren, Duchenne Parent Project, ZonMW, AFM and PPMD.He has been site principal investigator for clinical trials conducted by BioMarin, GSK, Eli Lilly, Santhera Pharmaceuticals, Italfarmaco SpA, Roche Pharma, Reveragen, NS Pharma, Fibrogen, Sarepta, Alexion, Janssen and Argnx outside the submitted work. He also reports ad hoc consultancies for BioMarin, Summit, PTC therapeutics, WAVE Life Sciences, Edgewise, Epirium Bio, Janssen, Sarepta and Regenxbio. All reimbursements were received by the LUMC. No personal financial benefits were received. Brenda Wong has participated in advisory committee meetings for Prosensa and Biomarin and has received compensation for consultancy services for Gilead Sciences, Pfizer, GSK and PepGen. Mirko Fillbrunn is an employee of Analysis Group, Inc., a consulting firm that received funding from the sponsors of cTAP to conduct this study. Gautam Sajeev is an employee of Analysis Group, Inc., a consulting firm that received funding from the sponsors of cTAP to conduct this study. Erica Yim was an employee of Analysis Group, Inc., a consulting firm that received funding from the sponsors of cTAP to conduct this study. Ibrahima Dieye was an employee of Analysis Group, Inc., a consulting firm that received funding from the sponsors of cTAP to conduct this study. Susan J. Ward co-founded and manages the collaborative Trajectory Analysis Project and has received funding from the sponsors of cTAP to facilitate this study. Nathalie Goemans has received compensation for consultancy services from Eli Lilly, Italfarmaco, PTC Therapeutics, BioMarin Pharmaceutical, Pfizer, Avidity, Daiichi Sankyo, Wave, Santhera and has served as site investigator for GlaxoSmithKline, Prosensa, BioMarin Pharmaceutical, Italfarmaco, Eli Lilly, Wave, and Sarepta. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
-
- McDonald CM, Henricson EK, Abresch RT, Duong T, Joyce NC, Hu F, et al.; CINRG Investigators. Long-term effects of glucocorticoids on function, quality of life, and survival in patients with Duchenne muscular dystrophy: a prospective cohort study. Lancet. 2018;391(10119):451–61. doi: 10.1016/S0140-6736(17)32160-8 - DOI - PubMed

