Association of Plasma Amyloid, P-Tau, GFAP, and NfL With CSF, Clinical, and Cognitive Features in Patients With Dementia With Lewy Bodies
- PMID: 38830138
- PMCID: PMC11244745
- DOI: 10.1212/WNL.0000000000209418
Association of Plasma Amyloid, P-Tau, GFAP, and NfL With CSF, Clinical, and Cognitive Features in Patients With Dementia With Lewy Bodies
Abstract
Background and objectives: Plasma β-amyloid-1-42/1-40 (Aβ42/40), phosphorylated-tau (P-tau), glial fibrillary acidic protein (GFAP), and neurofilament light (NfL) have been widely examined in Alzheimer disease (AD), but little is known about their reflection of copathologies, clinical importance, and predictive value in dementia with Lewy bodies (DLB). We aimed to evaluate associations of these biomarkers with CSF amyloid, cognition, and core features in DLB.
Methods: This cross-sectional multicenter cohort study with prospective component included individuals with DLB, AD, and healthy controls (HCs), recruited from 2002 to 2020 with an annual follow-up of up to 5 years, from the European-Dementia With Lewy Bodies consortium. Plasma biomarkers were measured by single-molecule array (Neurology 4-Plex E kit). Amyloid status was determined by CSF Aβ42 concentrations, and cognition was assessed by Mini-Mental State Examination (MMSE). Biomarker differences across groups, associations with amyloid status, and clinical core features were assessed by analysis of covariance. Associations with cognitive impairment and decline were assessed by linear regression and linear mixed-effects models.
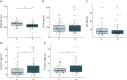
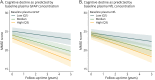
Results: In our cohort consisting of 562 individuals (HC n = 89, DLB n = 342, AD n = 131; 250 women [44.5%], mean [SD] age of 71 [8] years), sex distribution did not differ between groups. Patients with DLB were significantly older, and had less years of education and worse baseline cognition than HC, but not AD. DLB participants stratified for amyloid status differed significantly in plasma Aβ42/40 ratio (decreased in amyloid abnormal: β = -0.008, 95% CI -0.016 to -0.0003, p = 0.01) and P-tau (increased in amyloid abnormal, P-tau181: β = 0.246, 95% CI 0.011-0.481; P-tau231: β = 0.227, 95% CI 0.035-0.419, both p < 0.05), but not in GFAP (β = 0.068, 95% CI -0.018 to 0.153, p = 0.119), and NfL (β = 0.004, 95% CI -0.087 to 0.096, p = 0.923) concentrations. Higher baseline GFAP, NfL, and P-tau concentrations were associated with lower MMSE scores in DLB, and GFAP and NfL were associated with a faster cognitive decline (GFAP: annual change of -2.11 MMSE points, 95% CI -2.88 to -1.35 MMSE points, p < 0.001; NfL: annual change of -2.13 MMSE points, 95% CI -2.97 to -1.29 MMSE points, p < 0.001). DLB participants with parkinsonism had higher concentrations of NfL (β = 0.08, 95% CI 0.02-0.14, p = 0.006) than those without.
Discussion: Our study suggests a possible utility of plasma Aβ42/40, P-tau181, and P-tau231 as a noninvasive biomarkers to assess amyloid copathology in DLB, and plasma GFAP and NfL as monitoring biomarkers for cognitive symptoms in DLB.
Conflict of interest statement
K. Bolsewig, A.A.J.M. van Unnik, E.R. Blujdea, M.C. Gonzales, and N.J. Ashton report no disclosures relevant to the manuscript. D. Aarsland reports receiving research support and/or honoraria from AstraZeneca, H. Lundbeck, Novartis Pharmaceuticals, Biogen, Evonik, Sanofi, Roche, and GE Health and serving as a paid consultant for H. Lundbeck, Eisai, Heptares, and Mentis Cura. H. Zetterberg has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen, and Roche, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). A. Padovani reports no disclosures relevant to the manuscript. L. Bonanni reported receiving grants from the European Commission and the Italian Ministry of Health outside the submitted work. B. Mollenhauer reports no disclosures relevant to the manuscript. S. Schade received institutional salaries supported by the EU Horizon 2020 research and innovation program under grant agreement no. 863664 and by the Michael J. Fox Foundation for Parkinson's Research under grant agreement no. MJFF-021923. He is supported by a PPMI Early Stage Investigators Funding Program fellowship of the Michael J. Fox Foundation for Parkinson's Research under grant agreement no. MJFF-022656. R. Vandernberghe's institution has a clinical trial agreement (R. Vandenberghe as PI) with AviadoBio, Biogen, Denali, J&J, NovoNordisk, Prevail, Roche, UCB, Wave. R. Vandenberghe's institution has a consultancy agreement (R. Vandenberghe as a consultant) with ACImmune, Novartis, and Roche. K. Poesen and M.G. Kramberger report no disclosures relevant to the manuscript. C. Paquet reports serving as a member of the international advisory boards for Lilly; serving as a consultant for Fujiribio, Alzohis, Neuroimmune, Ads Neuroscience, Roche, AgenT, and Gilead; being involved as an investigator in several clinical trials for Roche, Eisai, Lilly, Biogen, AstraZeneca, Lundbeck, and Neuroimmune; and being a current member of the national boards of Roche, Lilly, and Biogen. O. Bousiges, B. Cretin, and E.A.J. Willemse report no disclosures relevant to the manuscript. C.E. Teunissen has a collaboration contract with ADx Neurosciences, Quanterix, and Eli Lilly, performed contract research or received grants from AC-Immune, Axon Neurosciences, BioConnect, Bioorchestra, Brainstorm Therapeutics, Celgene, EIP Pharma, Eisai, Fujirebio, Grifols, Instant Nano Biosensors, Merck, Novo Nordisk, PeopleBio, Roche, Siemens, Toyama, and Vivoryon. She is an editor of Alzheimer Research and Therapy, and serves on editorial boards of Medidact Neurologie/Springer, and Neurology: Neuroimmunology & Neuroinflammation. She had speaker contracts for Roche, Grifols, Novo Nordisk. A.W. Lemstra reports no disclosures relevant to the manuscript. Go to
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
