Peripuberty Is a Sensitive Period for Prefrontal Parvalbumin Interneuron Activity to Impact Adult Cognitive Flexibility
- PMID: 38830346
- PMCID: PMC11612032
- DOI: 10.1159/000539584
Peripuberty Is a Sensitive Period for Prefrontal Parvalbumin Interneuron Activity to Impact Adult Cognitive Flexibility
Abstract
Introduction: Developmental windows in which experiences can elicit long-lasting effects on brain circuitry and behavior are called "sensitive periods" and reflect a state of heightened plasticity. The classic example of a sensitive period comes from studies of sensory systems, like the visual system, where early visual experience is required for normal wiring of primary visual cortex and proper visual functioning. At a mechanistic level, loss of incoming visual input results in a decrease in activity in thalamocortical neurons representing the affected eye, resulting in an activity-dependent reduction in the representation of those inputs in the visual cortex and loss of visual perception in that eye. While associative cortical regions like the medial prefrontal cortex (mPFC) do not receive direct sensory input, recent findings demonstrate that changes in activity levels experienced by this region during defined windows in early development may also result in long-lasting changes in prefrontal cortical circuitry, network function, and behavior. For example, we recently demonstrated that decreasing the activity of mPFC parvalbumin-expressing (PV) interneurons during a period of time encompassing peripuberty (postnatal day P14) to adolescence (P50) led to a long-lasting decrease in their functional inhibition of pyramidal cells, as well as impairments in cognitive flexibility. While the effects of manipulating mPFC PV interneuron activity were selective to development, and not adulthood, the exact timing of the sensitive period for this manipulation remains unknown.
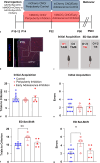
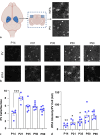
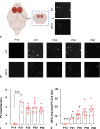
Methods: To refine the sensitive period in which inhibiting mPFC PV cell activity can lead to persistent effects on prefrontal functioning, we used a chemogenetic approach to restrict our inhibition of mPFC PV activity to two distinct windows: (1) peripuberty (P14-P32) and (2) early adolescence (P33-P50). We then investigated adult behavior after P90. In parallel, we performed histological analysis of molecular markers associated with sensitive period onset and offset in visual cortex, to define the onset and offset of peak-sensitive period plasticity in the mPFC.
Results: We found that inhibition of mPFC PV interneurons in peripuberty (P14-P32), but not adolescence (P33-P50), led to an impairment in set-shifting behavior in adulthood manifest as an increase in trials to reach criterion performance and errors. Consistent with a pubertal onset of sensitive period plasticity in the PFC, we found that histological markers of sensitive period onset and offset also demarcated P14 and P35, respectively. The time course of expression of these markers was similar in visual cortex.
Conclusion: Both lines of research converge on the peripubertal period (P14-P32) as one of heightened sensitive period plasticity in the mPFC. Further, our direct comparison of markers of sensitive period plasticity across the prefrontal and visual cortex suggests a similar time course of expression, challenging the notion that sensitive periods occur hierarchically. Together, these findings extend our knowledge about the nature and timing of sensitive period plasticity in the developing mPFC.
Introduction: Developmental windows in which experiences can elicit long-lasting effects on brain circuitry and behavior are called "sensitive periods" and reflect a state of heightened plasticity. The classic example of a sensitive period comes from studies of sensory systems, like the visual system, where early visual experience is required for normal wiring of primary visual cortex and proper visual functioning. At a mechanistic level, loss of incoming visual input results in a decrease in activity in thalamocortical neurons representing the affected eye, resulting in an activity-dependent reduction in the representation of those inputs in the visual cortex and loss of visual perception in that eye. While associative cortical regions like the medial prefrontal cortex (mPFC) do not receive direct sensory input, recent findings demonstrate that changes in activity levels experienced by this region during defined windows in early development may also result in long-lasting changes in prefrontal cortical circuitry, network function, and behavior. For example, we recently demonstrated that decreasing the activity of mPFC parvalbumin-expressing (PV) interneurons during a period of time encompassing peripuberty (postnatal day P14) to adolescence (P50) led to a long-lasting decrease in their functional inhibition of pyramidal cells, as well as impairments in cognitive flexibility. While the effects of manipulating mPFC PV interneuron activity were selective to development, and not adulthood, the exact timing of the sensitive period for this manipulation remains unknown.
Methods: To refine the sensitive period in which inhibiting mPFC PV cell activity can lead to persistent effects on prefrontal functioning, we used a chemogenetic approach to restrict our inhibition of mPFC PV activity to two distinct windows: (1) peripuberty (P14-P32) and (2) early adolescence (P33-P50). We then investigated adult behavior after P90. In parallel, we performed histological analysis of molecular markers associated with sensitive period onset and offset in visual cortex, to define the onset and offset of peak-sensitive period plasticity in the mPFC.
Results: We found that inhibition of mPFC PV interneurons in peripuberty (P14-P32), but not adolescence (P33-P50), led to an impairment in set-shifting behavior in adulthood manifest as an increase in trials to reach criterion performance and errors. Consistent with a pubertal onset of sensitive period plasticity in the PFC, we found that histological markers of sensitive period onset and offset also demarcated P14 and P35, respectively. The time course of expression of these markers was similar in visual cortex.
Conclusion: Both lines of research converge on the peripubertal period (P14-P32) as one of heightened sensitive period plasticity in the mPFC. Further, our direct comparison of markers of sensitive period plasticity across the prefrontal and visual cortex suggests a similar time course of expression, challenging the notion that sensitive periods occur hierarchically. Together, these findings extend our knowledge about the nature and timing of sensitive period plasticity in the developing mPFC.
Keywords: Adolescence; Parvalbumin; Peripuberty; Prefrontal cortex; Sensitive period.
© 2024 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
Similar articles
-
Timing of Methamphetamine Exposure during Adolescence Differentially Influences Parvalbumin and Perineuronal Net Immunoreactivity in the Medial Prefrontal Cortex of Female, but Not Male, Rats.Dev Neurosci. 2025;47(1):27-39. doi: 10.1159/000538608. Epub 2024 Mar 28. Dev Neurosci. 2025. PMID: 38547851 Free PMC article.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Antidepressants for pain management in adults with chronic pain: a network meta-analysis.Health Technol Assess. 2024 Oct;28(62):1-155. doi: 10.3310/MKRT2948. Health Technol Assess. 2024. PMID: 39367772 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Immunogenicity and seroefficacy of pneumococcal conjugate vaccines: a systematic review and network meta-analysis.Health Technol Assess. 2024 Jul;28(34):1-109. doi: 10.3310/YWHA3079. Health Technol Assess. 2024. PMID: 39046101 Free PMC article.
References
-
- Wiesel TN, Hubel DH. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol. 1963;26(6):1003–17. - PubMed
-
- Antonini A, Stryker MP. Plasticity of geniculocortical afferents following brief or prolonged monocular occlusion in the cat. J Comp Neurol. 1996;369(1):64–82. - PubMed
-
- Hubel DH, Wiesel TN, LeVay S. Functional architecture of area 17 in normal and monocularly deprived macaque monkeys. Cold Spring Harb Symp Quant Biol. 1976;40:581–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

