Accelerated Screening of Protein-Ligand Interactions via Parallel T2-Weighted 19F-MRI
- PMID: 38830623
- PMCID: PMC11190876
- DOI: 10.1021/acs.analchem.4c00333
Accelerated Screening of Protein-Ligand Interactions via Parallel T2-Weighted 19F-MRI
Abstract
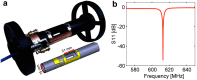
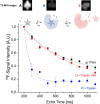
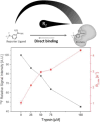
In drug discovery, ligands are sought that modulate the (mal-)function of medicinally relevant target proteins. In order to develop new drugs, typically a multitude of potential ligands are initially screened for binding and subsequently characterized for their affinity. Nuclear magnetic resonance (NMR) is a well-established and highly sensitive technology for characterizing such interactions. However, it has limited throughput, because only one sample can be measured at a time. In contrast, magnetic resonance imaging (MRI) is inherently parallel and MR parameters can conveniently be encoded in its images, potentially offering increased sample throughput. We explore this application using a custom-built 9-fold sample holder and a 19F-MRI coil. With this setup, we show that ligand binding can be detected by T2-weighted 19F-MRI using 4-(trifluoromethyl)benzamidine (TFBA) and trypsin as the reporter ligand and target protein, respectively. Furthermore, we demonstrate that the affinity of nonfluorinated ligands can be determined in a competition format by monitoring the dose-dependent displacement of TFBA. By comparing 19F-T2-weighted MR images of TFBA in the presence of different benzamidine (BA) concentrations-all recorded in parallel-the affinity of BA could be derived. Therefore, this approach promises parallel characterization of protein-ligand interactions and increased throughput of biochemical assays, with potential for increased sensitivity when combined with hyperpolarization techniques.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Determination of binding affinities using hyperpolarized NMR with simultaneous 4-channel detection.J Magn Reson. 2018 Oct;295:80-86. doi: 10.1016/j.jmr.2018.08.002. Epub 2018 Aug 13. J Magn Reson. 2018. PMID: 30144688 Free PMC article.
-
Polarization Transfer from Ligands Hyperpolarized by Dissolution Dynamic Nuclear Polarization for Screening in Drug Discovery.ChemMedChem. 2015 Sep;10(9):1559-63. doi: 10.1002/cmdc.201500241. Epub 2015 Aug 4. ChemMedChem. 2015. PMID: 26315550
-
Affinity screening using competitive binding with fluorine-19 hyperpolarized ligands.Angew Chem Int Ed Engl. 2015 Apr 13;54(16):4941-4. doi: 10.1002/anie.201411424. Epub 2015 Feb 20. Angew Chem Int Ed Engl. 2015. PMID: 25703090 Free PMC article.
-
NMR-based screening methods for lead discovery.EXS. 2003;(93):183-202. doi: 10.1007/978-3-0348-7997-2_9. EXS. 2003. PMID: 12613177 Review.
-
The role of magnetic resonance techniques in understanding and managing multiple sclerosis.Brain. 1998 Jan;121 ( Pt 1):3-24. doi: 10.1093/brain/121.1.3. Brain. 1998. PMID: 9549485 Review.
Cited by
-
Protein recognition methods for diagnostics and therapy.BBA Adv. 2025 Feb 14;7:100149. doi: 10.1016/j.bbadva.2025.100149. eCollection 2025. BBA Adv. 2025. PMID: 40060358 Free PMC article. Review.
-
Sensitive multichannel zero-to ultralow-field NMR with atomic magnetometer arrays.PNAS Nexus. 2025 Jun 27;4(6):pgaf187. doi: 10.1093/pnasnexus/pgaf187. eCollection 2025 Jun. PNAS Nexus. 2025. PMID: 40583910 Free PMC article.
References
-
- Macarron R.; Banks M. N.; Bojanic D.; Burns D. J.; Cirovic D. A.; Garyantes T.; Green D. V. S.; Hertzberg R. P.; Janzen W. P.; Paslay J. W.; Schopfer U.; Sittampalam G. S. Impact of high-throughput screening in biomedical research. Nat. Rev. Drug Discovery 2011, 10, 188–195. 10.1038/nrd3368. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

