Rab6-Mediated Polarized Transport of Synaptic Vesicle Precursors Is Essential for the Establishment of Neuronal Polarity and Brain Formation
- PMID: 38830762
- PMCID: PMC11223463
- DOI: 10.1523/JNEUROSCI.2334-23.2024
Rab6-Mediated Polarized Transport of Synaptic Vesicle Precursors Is Essential for the Establishment of Neuronal Polarity and Brain Formation
Abstract
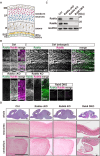
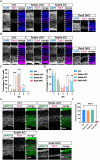
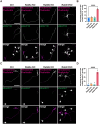
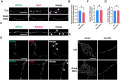
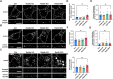
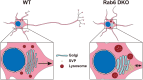
Neurons are highly polarized cells that are composed of a single axon and multiple dendrites. Axon-dendrite polarity is essential for proper tissue formation and brain functions. Intracellular protein transport plays an important role in the establishment of neuronal polarity. However, the regulatory mechanism of polarized transport remains unclear. Here, we show that Rab6, a small GTPase that acts on the regulation of intracellular vesicular trafficking, plays key roles in neuronal polarization and brain development. Central nervous system-specific Rab6a/b double knock-out (Rab6 DKO) mice of both sexes exhibit severe dysplasia of the neocortex and the cerebellum. In the Rab6 DKO neocortex, impaired axonal extension of neurons results in hypoplasia of the intermediate zone. In vitro, deletion of Rab6a and Rab6b in cultured neurons from both sexes causes the abnormal accumulation of synaptic vesicle precursors (SVPs) adjacent to the Golgi apparatus, which leads to defects in axonal extension and the loss of axon-dendrite polarity. Moreover, Rab6 DKO causes significant expansion of lysosomes in the soma in neurons. Overall, our results reveal that Rab6-mediated polarized transport of SVPs is crucial for neuronal polarization and subsequent brain formation.
Keywords: Golgi apparatus; Rab6; cell polarity; knock-out mouse; synaptic vesicle.
Copyright © 2024 the authors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures







Similar articles
-
RAB6 and dynein drive post-Golgi apical transport to prevent neuronal progenitor delamination.EMBO Rep. 2022 Oct 6;23(10):e54605. doi: 10.15252/embr.202254605. Epub 2022 Aug 18. EMBO Rep. 2022. PMID: 35979738 Free PMC article.
-
The small GTPase Rab6B, a novel Rab6 subfamily member, is cell-type specifically expressed and localised to the Golgi apparatus.J Cell Sci. 2000 Aug;113 ( Pt 15):2725-35. doi: 10.1242/jcs.113.15.2725. J Cell Sci. 2000. PMID: 10893188
-
Structural basis of ELKS/Rab6B interaction and its role in vesicle capturing enhanced by liquid-liquid phase separation.J Biol Chem. 2023 Jun;299(6):104808. doi: 10.1016/j.jbc.2023.104808. Epub 2023 May 11. J Biol Chem. 2023. PMID: 37172719 Free PMC article.
-
Critical importance of RAB proteins for synaptic function.Small GTPases. 2018 Mar 4;9(1-2):145-157. doi: 10.1080/21541248.2016.1277001. Epub 2017 Apr 13. Small GTPases. 2018. PMID: 28146371 Free PMC article. Review.
-
Structural aspects of Rab6-effector complexes.Biochem Soc Trans. 2009 Oct;37(Pt 5):1037-41. doi: 10.1042/BST0371037. Biochem Soc Trans. 2009. PMID: 19754447 Review.
Cited by
-
Molecular mechanisms of polarized transport to the apical plasma membrane.Front Cell Dev Biol. 2024 Sep 26;12:1477173. doi: 10.3389/fcell.2024.1477173. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39445332 Free PMC article. Review.
-
The neuronal Golgi in neural circuit formation and reorganization.Front Neural Circuits. 2024 Dec 5;18:1504422. doi: 10.3389/fncir.2024.1504422. eCollection 2024. Front Neural Circuits. 2024. PMID: 39703196 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
