Neutral or Detrimental Effects of TREM2 Agonist Antibodies in Preclinical Models of Alzheimer's Disease and Multiple Sclerosis
- PMID: 38830764
- PMCID: PMC11255434
- DOI: 10.1523/JNEUROSCI.2347-23.2024
Neutral or Detrimental Effects of TREM2 Agonist Antibodies in Preclinical Models of Alzheimer's Disease and Multiple Sclerosis
Abstract
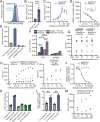
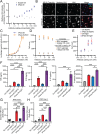
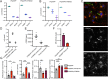
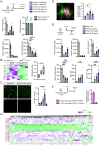
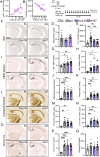
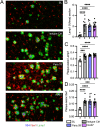
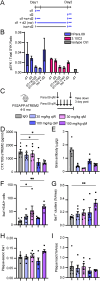
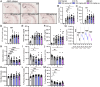
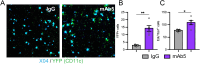
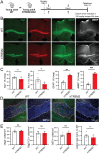
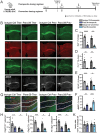
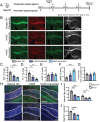
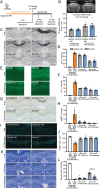
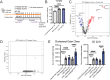
Human genetics and preclinical studies have identified key contributions of TREM2 to several neurodegenerative conditions, inspiring efforts to modulate TREM2 therapeutically. Here, we characterize the activities of three TREM2 agonist antibodies in multiple mixed-sex mouse models of Alzheimer's disease (AD) pathology and remyelination. Receptor activation and downstream signaling are explored in vitro, and active dose ranges are determined in vivo based on pharmacodynamic responses from microglia. For mice bearing amyloid-β (Aβ) pathology (PS2APP) or combined Aβ and tau pathology (TauPS2APP), chronic TREM2 agonist antibody treatment had limited impact on microglia engagement with pathology, overall pathology burden, or downstream neuronal damage. For mice with demyelinating injuries triggered acutely with lysolecithin, TREM2 agonist antibodies unexpectedly disrupted injury resolution. Likewise, TREM2 agonist antibodies limited myelin recovery for mice experiencing chronic demyelination from cuprizone. We highlight the contributions of dose timing and frequency across models. These results introduce important considerations for future TREM2-targeting approaches.
Keywords: Alzheimer’s disease; TREM2; antibody; microglia; multiple sclerosis; myelin repair.
Copyright © 2024 the authors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
