Trifluridine/tipiracil with and without ramucirumab for advanced gastric cancer: a comparative observational study
- PMID: 38830895
- PMCID: PMC11148118
- DOI: 10.1038/s41598-024-61975-7
Trifluridine/tipiracil with and without ramucirumab for advanced gastric cancer: a comparative observational study
Abstract
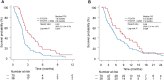
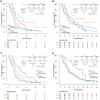
The combination of trifluridine/tipiracil hydrochloride (FTD/TPI) plus ramucirumab has demonstrated clinical activity in patients with advanced gastric cancer (AGC). We evaluated the efficacy and safety of this combination compared with those of FTD/TPI monotherapy in patients with AGC. We retrospectively reviewed data of patients with AGC who received FTD/TPI plus ramucirumab or FTD/TPI monotherapy as third- or later-line treatment. This study included 36 patients treated with FTD/TPI plus ramucirumab and 70 patients receiving FTD/TPI monotherapy. The objective response rate (ORR) and disease control rate (DCR) were 25.8% and 58.1%, respectively, in the FTD/TPI plus ramucirumab group and 5.0% and 38.3%, respectively, in the FTD/TPI group (ORR, P = 0.007; DCR, P = 0.081). The median progression-free survival (PFS) was significantly longer in the FTD/TPI plus ramucirumab group (median PFS, 2.9 vs. 1.8 months; hazard ratio [HR]: 0.52; P = 0.001). A numerical survival benefit was also observed (median overall survival, 7.9 months vs. 5.0 months; HR: 0.68, P = 0.089). In the multivariate analysis, PFS was significantly longer in the FTD/TPI plus ramucirumab group than in the FTD/TPI monotherapy group (HR: 0.61, P = 0.030). The incidence of febrile neutropenia was higher in the FTD/TPI plus ramucirumab group than in the FTD/TPI group (13.8% vs. 2.9%); however, no new safety signals were identified. Compared with FTD/TPI monotherapy, FTD/TPI plus ramucirumab offers clinical benefits with acceptable toxicity in heavily pretreated patients with AGC. Further investigation via randomized trials is warranted to confirm these findings.
Keywords: Chemotherapy; Gastric cancer; Ramucirumab; Trifluridine/tipiracil.
© 2024. The Author(s).
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered potential competing interests: Y Narita: research funding to my institution from Chugai, MSD, Amgen, ONO Pharmaceutical, Astellas, Sanofi, Taiho, Eisai, Daiichi Sankyo, Novartis, and Pfizer; honoraria for lectures, presentations, and speaker bureaus from Yakult Honsha, Taiho, Eli Lilly, Daiichi Sankyo, Ono Pharma, and Bristol-Meyers Squibb and; participation on the Advisory Board of Daiichi Sankyo. T Ogata: personal fees from ONO Pharmaceutical, personal fees from BMS, personal fees from Taiho, personal fees from Daichi-Sankyo, outside the submitted work. H Kodama: honoraria for lectures, presentations, and speaker bureaus from Taiho. K Honda: grants from Pfizer, outside the submitted work; T Masuishi: grants and personal fees from MSD, grants and personal fees from Amgen, grants and personal fees from ONO Pharmaceutical, grants and personal fees from Daiichi Sankyo, grants from Novartis, grants from Pfizer, personal fees from Taiho, personal fees from Bristol Myers Squibb, personal fees from Eli Lilly, personal fees from Takeda, grants from Boehringer-Ingelheim, grants from Syneos Healthe Clinical, personal fees from Chugai, personal fees from Nippon Kayaku, grants from Cimic Shift Zero, personal fees from Merck Bio Pharma, personal fees from Bayer, personal fees from Yakult Honsha, personal fees from Sanofi, personal fees from ONO Pharmaceutical, outside the submitted work. H Taniguchi: grants and personal fees from Takeda, grants from Daiichi Sankyo, grants and personal fees from ONO Pharmaceutical, personal fees from Eli Lilly, personal fees from Merck Biopharma, personal fees from Chugai, outside the submitted work; Dr. Kadowaki reports grants and personal fees from Eli Lilly, grants from Nobelpharma, grants and personal fees from Taiho, grants and personal fees from MSD, grants and personal fees from Bayer, grants from Yansen, grants and personal fees from Chugai, grants and personal fees from ONO Pharmaceutical, grants from Daiichi Sankyo, personal fees from Merck, personal fees from BMS, outside the submitted work; S Kadowaki: Grants or contracts from Eli Lilly, Ono, Bayer,Daiichi Sankyo, MSD, Chugai, Janssen, Nobelpharma, payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Bristol-Myers Squibb, Ono, Bayer, Merck Biopharma, Taiho, Eisai, Daiichi Sankyo, MSD, Chugai, Otsuka Pharmaceutical. M Ando: personal fees from Eisai Co., Ltd., personal fees from ONO Pharmaceutical, personal fees from Chugai Pharmaceutical Co. Ltd, personal fees from Mundipharma Co., Ltd., personal fees from Taiho Pharmaceutical Co., Ltd., outside the submitted work. M Tajika: personal fees from Bristol Myers Squibb, outside the submitted work. K Muro: research funding to my institution from Chugai, MSD, Amgen, ONO Pharmaceutical, Astellas, Sanofi, Taiho, Eisai, Daiichi Sankyo, Novartis, and Pfizer; consulting fees from AstraZeneca, ONO Pharmaceutical, Amgen, and Astellas; honoraria for lectures from ONO Pharmaceutical, Taiho, Bristol-Myers Squibb, Eli Lilly, MSD, and Daiichi Sankyo; participation on the advisory boards of ONO Pharmaceutical, Amgen, AstraZeneca, Eli Lilly, and Takeda.
Figures

References
-
- Komatsu Y, Hironaka S, Tanizawa Y, Cai Z, Piao Y, Boku N. Treatment pattern for advanced gastric cancer in Japan and factors associated with sequential treatment: A retrospective administrative claims database study. Adv. Ther. 2022;39(1):296–313. doi: 10.1007/s12325-021-01931-3. - DOI - PMC - PubMed
-
- Shitara K, Doi T, Dvorkin M, Mansoor W, Arkenau HT, Prokharau A, et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19(11):1437–1448. doi: 10.1016/S1470-2045(18)30739-3. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

