Differential responses of primary neuron-secreted MCP-1 and IL-9 to type 2 diabetes and Alzheimer's disease-associated metabolites
- PMID: 38830911
- PMCID: PMC11148169
- DOI: 10.1038/s41598-024-62155-3
Differential responses of primary neuron-secreted MCP-1 and IL-9 to type 2 diabetes and Alzheimer's disease-associated metabolites
Abstract
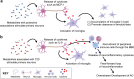
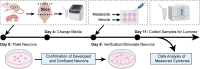
Type 2 diabetes (T2D) is implicated as a risk factor for Alzheimer's disease (AD), the most common form of dementia. In this work, we investigated neuroinflammatory responses of primary neurons to potentially circulating, blood-brain barrier (BBB) permeable metabolites associated with AD, T2D, or both. We identified nine metabolites associated with protective or detrimental properties of AD and T2D in literature (lauric acid, asparagine, fructose, arachidonic acid, aminoadipic acid, sorbitol, retinol, tryptophan, niacinamide) and stimulated primary mouse neuron cultures with each metabolite before quantifying cytokine secretion via Luminex. We employed unsupervised clustering, inferential statistics, and partial least squares discriminant analysis to identify relationships between cytokine concentration and disease-associations of metabolites. We identified MCP-1, a cytokine associated with monocyte recruitment, as differentially abundant between neurons stimulated by metabolites associated with protective and detrimental properties of AD and T2D. We also identified IL-9, a cytokine that promotes mast cell growth, to be differentially associated with T2D. Indeed, cytokines, such as MCP-1 and IL-9, released from neurons in response to BBB-permeable metabolites associated with T2D may contribute to AD development by downstream effects of neuroinflammation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Update of
-
Differential responses of primary neuron-secreted MCP-1 and IL-9 to type 2 diabetes and Alzheimer's disease-associated metabolites.bioRxiv [Preprint]. 2023 Nov 17:2023.11.17.567595. doi: 10.1101/2023.11.17.567595. bioRxiv. 2023. Update in: Sci Rep. 2024 Jun 3;14(1):12743. doi: 10.1038/s41598-024-62155-3. PMID: 38014333 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

