Lisocabtagene maraleucel in follicular lymphoma: the phase 2 TRANSCEND FL study
- PMID: 38830991
- PMCID: PMC11333271
- DOI: 10.1038/s41591-024-02986-9
Lisocabtagene maraleucel in follicular lymphoma: the phase 2 TRANSCEND FL study
Erratum in
-
Author Correction: Lisocabtagene maraleucel in follicular lymphoma: the phase 2 TRANSCEND FL study.Nat Med. 2024 Aug;30(8):2374. doi: 10.1038/s41591-024-03175-4. Nat Med. 2024. PMID: 38982297 Free PMC article. No abstract available.
Abstract
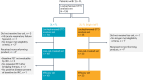
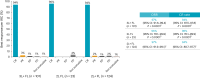
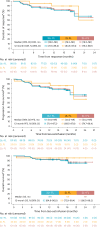
An unmet need exists for patients with relapsed/refractory (R/R) follicular lymphoma (FL) and high-risk disease features, such as progression of disease within 24 months (POD24) from first-line immunochemotherapy or disease refractory to both CD20-targeting agent and alkylator (double refractory), due to no established standard of care and poor outcomes. Chimeric antigen receptor (CAR) T cell therapy is an option in R/R FL after two or more lines of prior systemic therapy, but there is no consensus on its optimal timing in the disease course of FL, and there are no data in second-line (2L) treatment of patients with high-risk features. Lisocabtagene maraleucel (liso-cel) is an autologous, CD19-directed, 4-1BB CAR T cell product. The phase 2 TRANSCEND FL study evaluated liso-cel in patients with R/R FL, including 2L patients who all had POD24 from diagnosis after treatment with anti-CD20 antibody and alkylator ≤6 months of FL diagnosis and/or met modified Groupe d'Etude des Lymphomes Folliculaires criteria. Primary/key secondary endpoints were independent review committee-assessed overall response rate (ORR)/complete response (CR) rate. At data cutoff, 130 patients had received liso-cel (median follow-up, 18.9 months). Primary/key secondary endpoints were met. In third-line or later FL (n = 101), ORR was 97% (95% confidence interval (CI): 91.6‒99.4), and CR rate was 94% (95% CI: 87.5‒97.8). In 2L FL (n = 23), ORR was 96% (95% CI: 78.1‒99.9); all responders achieved CR. Cytokine release syndrome occurred in 58% of patients (grade ≥3, 1%); neurological events occurred in 15% of patients (grade ≥3, 2%). Liso-cel demonstrated efficacy and safety in patients with R/R FL, including high-risk 2L FL. ClinicalTrials.gov identifier: NCT04245839 .
© 2024. The Author(s).
Conflict of interest statement
F.M. discloses consultancy for AbbVie, Bristol-Myers Squibb, Genmab, Gilead, Novartis and Roche and service as an advisor for AbbVie, Gilead and Roche. S.D. discloses service as an advisor to Bristol-Myers Squibb and Kite/Gilead and research funding from Kite/Gilead. M.L.P. discloses honoraria from Cellectar Biosciences, Ceramedix, Juno Therapeutics, Garuda Therapeutics, Kite, Mustang Bio, Novartis, Pluto Immunotherapeutics, Rheos Medicines, Seres Therapeutics, Smart Immune, Synthekine and ThymoFox and royalties from Juno Therapeutics and Seres Therapeutics. A.M.G.-S. discloses consultancy for AbbVie, ADC Therapeutics America, Celgene, Clinigen, EUSA Pharma, Gilead/Kite, Ideogen, Incyte, Kyowa Kirin, Eli Lilly, Miltenyi Biotec, Novartis, Roche and Takeda; honoraria from Celgene, EUSA Pharma, Gilead/Kite, Janssen, Novartis, Roche and Takeda; travel from Celgene, Gilead/Kite, Janssen and Roche; and service as an advisor for Bristol-Myers Squibb. J.L.R.O. discloses consultancy for Janssen and honoraria from Amgen, Bristol-Myers Squibb and Kite. J. Kuruvilla discloses consultancy for AbbVie, Bristol-Myers Squibb, Gilead, Merck, Roche and Seattle Genetics; honoraria from AbbVie, Amgen, AstraZeneca, Bristol-Myers Squibb, Genmab, Gilead, Incyte, Janssen, Merck, Novartis, Pfizer, Roche and Seattle Genetics; service as an advisor for Karyopharm Therapeutics; and research funding from AstraZeneca, Merck and Roche. U.J. discloses honoraria from Bristol-Myers Squibb, Gilead, Janssen, Miltenyi Biotec, Novartis and Roche and research funding from Innovative Medicines Initiative 2 Joint Undertaking. G.C. discloses consultancy for AbbVie, Bristol-Myers Squibb, Emercell, MabQi, MedXCell, Onward Therapeutics and Roche and honoraria from AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Novartis and Roche. K.I. discloses consultancy for AbbVie, AstraZeneca, Eisai, Genmab, Mitsubishi Tanabe Pharma, Ono Pharmaceutical, Otsuka, Nihon Shinyaku, Takeda and Zenyaku; honoraria from AbbVie, AstraZeneca, Bristol-Myers Squibb, Chugai, Daiichi Sankyo, Eisai, Genmab, Janssen, Kyowa Kirin, Eli Lilly, Merck Sharp & Dohme, Nihon Kayaku, Novartis, Ono Pharmaceutical, Pfizer, SymBio Pharmaceuticals, Seika Pharma and Takeda; and research funding from AbbVie, Astellas, Amgen, AstraZeneca, BeiGene, Bristol-Myers Squibb, Chugai, Daiichi Sankyo, Eisai, Genmab, Incyte, Kyowa Kirin, Loxo Oncology, Merck Sharp & Dohme, Novartis, Otsuka, Pfizer, Regeneron and Yakult. M.D. discloses honoraria from AstraZeneca, BeiGene, Gilead/Kite, Janssen, Eli Lilly, Novartis and Roche; service as an advisor for AbbVie, AstraZeneca, BeiGene, Celgene, Gilead/Kite, Janssen, Eli Lilly/Loxo, Novartis and Roche; and research funding from AbbVie, Bayer, Celgene, Gilead/Kite, Janssen and Roche. B.K. discloses honoraria from Bristol-Myers Squibb Germany and MSD Oncology and research funding from MSD Oncology and Takeda. H. Ghesquieres discloses consultancy for Gilead and Roche and honoraria from AbbVie, Bristol-Myers Squibb, Gilead and Roche. K.A. discloses travel from Bristol-Myers Squibb, Gilead and Novartis. H. Goto discloses honoraria from Bristol-Myers Squibb, Chugai, Novartis, AbbVie, Gilead/Kite, Merck Sharp & Dohme, Daiichi Sankyo, Eisai, Kyowa Kirin and SymBio and research funding from Bristol-Myers Squibb, Kyowa Kirin, Sanofi and SymBio. A.M.B. and S.d.V. declare no competing interests. J.S.A. discloses consultancy for AbbVie, Alimera Sciences, AstraZeneca, BeiGene, bluebird bio, C4 Therapeutics, Caribou Biosciences, Celgene, Century Therapeutics, EMD Serono, Epizyme, Genentech, Genmab, Incyte, Janssen, Karyopharm Therapeutics, Kite/Gilead, Kymera Therapeutics, Eli Lilly, MorphoSys, Mustang Bio, Novartis, Ono Pharmaceutical and Takeda; honoraria from AstraZeneca, Bristol-Myers Squibb, Janssen and Regeneron; and research funding from AI Therapeutics, Bristol-Myers Squibb and Seattle Genetics. P.B. discloses honoraria from Bristol-Myers Squibb Germany and MSD Oncology and research funding from MSD Oncology and Takeda. I.F. discloses consultancy for AbbVie, AstraZeneca, BeiGene, Bristol-Myers Squibb, Gilead, Incyte, Janssen, Novartis, Roche and Seagen and honoraria from Gilead, Incyte, Novartis and Seagen. S.M. discloses honoraria from Celgene, Novartis, Gilead/Kite, Janssen, JAMP Pharma and Pfizer (via institution); service as part of a data safety monitoring board for Immunicum/Mendes and Miltenyi Biotec (via institution); and founding leadership for SWECARNET (via institution) and founder for ScientifyResearch (spouse). A.S. discloses consultancy for Alexion Pharmaceuticals, AstraZeneca, Bristol-Myers Squibb, Epizyme, Genentech, Genmab, Janssen, Kite, Eli Lilly, MorphoSys, Novartis, Pharmacyclics, Seagen and TG Therapeutics and honoraria from AbbVie, ADC Therapeutics, Alexion Pharmaceuticals, AstraZeneca, BeiGene, Epizyme, Genentech, Genmab, Janssen, Jazz Pharmaceuticals, Kite, MorphoSys, Pharmacyclics, Seagen and TG Therapeutics. M.K. discloses consultancy for AbbVie, Adaptive Biotechnologies, ADC Therapeutics, AstraZeneca, BeiGene, Caribou Biosciences, Celgene, Genentech and Syncopation; service as an advisor for Celgene, Genentech and Seagen; and research funding from Novartis. R.K. discloses consultancy for AstraZeneca, BeiGene, Bristol-Myers Squibb, Calithera Biosciences, Epizyme, Genentech, Janssen, Kite/Gilead, Eli Lilly Oncology, Miltenyi Biotec and MorphoSys; honoraria from AstraZeneca, BeiGene and MorphoSys; and other interests with Bristol-Myers Squibb, Calithera Biosciences, Kite/Gilead and Miltenyi Biotec. A.V. discloses consultancy for AbbVie, Bristol-Myers Squibb, Kite/Gilead and Roche; honoraria from AbbVie, Bristol-Myers Squibb, Kite/Gilead and Roche; and research funding from Bristol-Myers Squibb. T.F., O.F., J.L., M.V., R.N., A.A., J.P. and J. Kumar are employees and equity holders of Bristol-Myers Squibb. L.J.N. discloses consultancy for Interius Biotherapeutics and SIRPant Immunotherapeutics; honoraria from ADC Therapeutics, Bristol-Myers Squibb, Caribou Biosciences, Epizyme, Genentech, Genmab, Gilead, Janssen Oncology, MorphoSys, Novartis, Roche, Takeda and TG Therapeutics; and travel from Genentech/Roche.
Figures
References
-
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). B-Cell Lymphomas. Version 1.2024. https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf (National Comprehensive Cancer Network, 2024).
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

