Isatuximab, lenalidomide, dexamethasone and bortezomib in transplant-ineligible multiple myeloma: the randomized phase 3 BENEFIT trial
- PMID: 38830994
- PMCID: PMC11333283
- DOI: 10.1038/s41591-024-03050-2
Isatuximab, lenalidomide, dexamethasone and bortezomib in transplant-ineligible multiple myeloma: the randomized phase 3 BENEFIT trial
Abstract
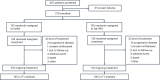
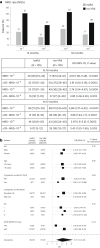
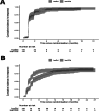
CD38-targeting immunotherapy is approved in combination with lenalidomide and dexamethasone in patients with newly diagnosed multiple myeloma (NDMM) that are transplant ineligible (TI) and is considered the best standard of care (SOC). To improve current SOC, we evaluated the added value of weekly bortezomib (V) to isatuximab plus lenalidomide and dexamethasone (IsaRd versus Isa-VRd). This Intergroupe Francophone of Myeloma phase 3 study randomized 270 patients with NDMM that were TI, aged 65-79 years, to IsaRd versus Isa-VRd arms. The primary endpoint was a minimal residual disease (MRD) negativity rate at 10-5 by next-generation sequencing at 18 months from randomization. Key secondary endpoints included response rates, MRD assessment rates, survival and safety. The 18-month MRD negativity rates at 10-5 were reported in 35 patients (26%, 95% confidence interval (CI) 19-34) in IsaRd versus 71 (53%, 95% CI 44-61) in Isa-VRd (odds ratio for MRD negativity 3.16, 95% CI 1.89-5.28, P < 0.0001). The MRD benefit was consistent across subgroups at 10-5 and 10-6, and was already observed at month 12. The proportion of patients with complete response or better at 18 months was higher with Isa-VRd (58% versus 33%; P < 0.0001), as was the proportion of MRD negativity and complete response or better (37% versus 17%; P = 0.0003). At a median follow-up of 23.5 months, no difference was observed for survival times (immature data). The addition of weekly bortezomib did not significantly affect the relative dose intensity of IsaRd. Isa-VRd significantly increased MRD endpoints, including the 18-month negativity rate at 10-5, the primary endpoint, compared with IsaRd. This study proposes Isa-VRd as a new SOC for patients with NDMM that are TI. ClinicalTrials.gov identifier: NCT04751877 .
© 2024. The Author(s).
Conflict of interest statement
The authors report the following competing interests. X.L. has received consultancy, honoraria and travel fees from Sanofi, Janssen-Cilag, Kite/Gilead, Amgen, Novartis, Takeda, Pfizer, Oncopeptide, AbbVie, GSK and Bristol Myers Squibb. C.H. reports consultancy and honoraria from Janssen-Cilag. A.B. received consultancy and honoraria from Sanofi, Janssen-Cilag. A.P. reports receiving consultancy, honoraria and travel fees from AbbVie, Amgen, Bristol Myers Squibb, Janssen, GSK, Menarini Stemline, Pfizer, Sanofi and Takeda. L.K. has received consultancy, honoraria and travel fees from Sanofi, Janssen-Cilag, Kite/Gilead, Amgen, Novartis, Takeda, Pfizer, Oncopeptide, AbbVie, GSK and Bristol Myers Squibb. T.C. reports receiving consultancy fees and honoraria from Sanofi and Janssen-Cilag. C.T. has received consultancy, honoraria and travel fees from Sanofi, Janssen-Cilag, Kite/Gilead, Amgen, Novartis, Takeda, Pfizer, Oncopeptide, AbbVie, GSK and Bristol Myers Squibb. M.M. has received consultancy, honoraria and travel fees from Sanofi, Janssen-Cilag, Kite/Gilead, Amgen, Novartis, Takeda, Pfizer, Oncopeptide, AbbVie, GSK and Bristol Myers Squibb. P.M. reports consultancy fees and honoraria from Janssen-Cilag, Amgen, Novartis, Takeda, Pfizer, AbbVie, GSK, Bristol Myers Squibb and Sanofi. H.AL. has received consultancy, honoraria and travel fees from Janssen, Sanofi, Bristol Myers Squibb, Pfizer and Adaptive, and research support from Sanofi and Bristol Myers Squibb. J.C. reports consultancy, honoraria and travel fees from Janssen, Sanofi, Bristol Myers Squibb, Pfizer and Adaptive, and research support from Sanofi and Bristol Myers Squibb. The other authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

