New molecular and macroscopic understandings of novel green chemicals based on Xanthan Gum and bio-surfactants for enhanced oil recovery
- PMID: 38831003
- PMCID: PMC11148032
- DOI: 10.1038/s41598-024-63244-z
New molecular and macroscopic understandings of novel green chemicals based on Xanthan Gum and bio-surfactants for enhanced oil recovery
Abstract
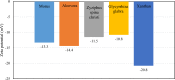
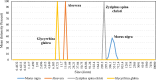
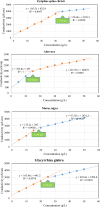
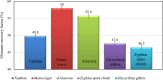
This research investigates the interactions between a novel environmentally friendly chemical fluid consisting of Xanthan gum and bio-based surfactants, and crude oil. The surfactants, derived from various leaves using the spray drying technique, were characterized using Fourier-transform infrared (FTIR) spectroscopy, zeta potential analysis, Dynamic light scattering, and evaluation of critical micelle concentration. Static emulsion tests were conducted to explore the emulsification between crude oil and the polymer-surfactant solution. Analysis of the bulk oil FTIR spectra revealed that saturated hydrocarbons and light aromatic hydrocarbons exhibited a higher tendency to adsorb onto the emulsion phase. Furthermore, the increased presence of polar hydrocarbons in emulsion phases generated by polar surfactants confirmed the activation of electrostatic forces in fluid-fluid interactions. Nuclear magnetic resonance spectroscopy showed that the xanthan solution without surfactants had a greater potential to adsorb asphaltenes with highly fused aromatic rings, while the presence of bio-based surfactants reduced the solution's ability to adsorb asphaltenes with larger cores. Microfluidic tests demonstrated that incorporating surfactants derived from Morus nigra and Aloevera leaves into the xanthan solution enhanced oil recovery. While injection of the xanthan solution resulted in a 49.8% recovery rate, the addition of Morus nigra and Aloevera leaf-derived surfactants to the xanthan solution increased oil recovery to 58.1% and 55.8%, respectively.
Keywords: Asphaltene; Leaf-derived surfactants; Microfluidic Injections; Molecular characterization; Xanthan Gum.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Salehi N, Saeedi Dehaghani A, Haghighi M. Investigation of fluid-fluid interaction between surfactant-ion-tuned water and crude oil: A new insight into asphaltene behavior in the emulsion interface. J. Mol. Liq. 2023;376:121311. doi: 10.1016/j.molliq.2023.121311. - DOI
-
- Nourani, M. & Sadeghnejad, S. Alkaline-surfactant polymer (ASP). in Chemical Methods 821931 (2022).
-
- Tajikmansori A, Hosseini M, Dehaghani AHS. Mechanistic study to investigate the injection of surfactant assisted smart water in carbonate rocks for enhanced oil recovery: An experimental approach. J. Mol. Liq. 2021;325:114648. doi: 10.1016/j.molliq.2020.114648. - DOI
LinkOut - more resources
Full Text Sources

