LORIS robustly predicts patient outcomes with immune checkpoint blockade therapy using common clinical, pathologic and genomic features
- PMID: 38831056
- PMCID: PMC11962634
- DOI: 10.1038/s43018-024-00772-7
LORIS robustly predicts patient outcomes with immune checkpoint blockade therapy using common clinical, pathologic and genomic features
Abstract
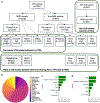
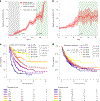
Despite the revolutionary impact of immune checkpoint blockade (ICB) in cancer treatment, accurately predicting patient responses remains challenging. Here, we analyzed a large dataset of 2,881 ICB-treated and 841 non-ICB-treated patients across 18 solid tumor types, encompassing a wide range of clinical, pathologic and genomic features. We developed a clinical score called LORIS (logistic regression-based immunotherapy-response score) using a six-feature logistic regression model. LORIS outperforms previous signatures in predicting ICB response and identifying responsive patients even with low tumor mutational burden or programmed cell death 1 ligand 1 expression. LORIS consistently predicts patient objective response and short-term and long-term survival across most cancer types. Moreover, LORIS showcases a near-monotonic relationship with ICB response probability and patient survival, enabling precise patient stratification. As an accurate, interpretable method using a few readily measurable features, LORIS may help improve clinical decision-making in precision medicine to maximize patient benefit. LORIS is available as an online tool at https://loris.ccr.cancer.gov/ .
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Competing interests
E.R. is a cofounder of MedAware, Metabomed and Pangea Biomed (divested) and an unpaid member of Pangea Biomed’s scientific advisory board. L.G.T.M. is listed as an inventor on intellectual property owned by MSK related to the use of TMB in cancer immunotherapy, unrelated to this work. The other authors declare no competing interests.
Figures















References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

