Tumor-associated macrophages restrict CD8+ T cell function through collagen deposition and metabolic reprogramming of the breast cancer microenvironment
- PMID: 38831058
- PMCID: PMC12204312
- DOI: 10.1038/s43018-024-00775-4
Tumor-associated macrophages restrict CD8+ T cell function through collagen deposition and metabolic reprogramming of the breast cancer microenvironment
Abstract
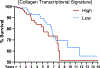
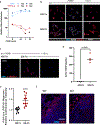
Tumor progression is accompanied by fibrosis, a condition of excessive extracellular matrix accumulation, which is associated with diminished antitumor immune infiltration. Here we demonstrate that tumor-associated macrophages (TAMs) respond to the stiffened fibrotic tumor microenvironment (TME) by initiating a collagen biosynthesis program directed by transforming growth factor-β. A collateral effect of this programming is an untenable metabolic milieu for productive CD8+ T cell antitumor responses, as collagen-synthesizing macrophages consume environmental arginine, synthesize proline and secrete ornithine that compromises CD8+ T cell function in female breast cancer. Thus, a stiff and fibrotic TME may impede antitumor immunity not only by direct physical exclusion of CD8+ T cells but also through secondary effects of a mechano-metabolic programming of TAMs, which creates an inhospitable metabolic milieu for CD8+ T cells to respond to anticancer immunotherapies.
© 2024. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures











References
MeSH terms
Substances
Grants and funding
- R01CA192914/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- R01 CA192914/CA/NCI NIH HHS/United States
- S10 OD016387/OD/NIH HHS/United States
- R01 NS109911/NS/NINDS NIH HHS/United States
- P30 CA014599/CA/NCI NIH HHS/United States
- R01 CA222508/CA/NCI NIH HHS/United States
- R35 CA242447-01A1/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- 1F32CA236156-01A1/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- R35 CA242447/CA/NCI NIH HHS/United States
- F32 CA236156/CA/NCI NIH HHS/United States
- U01 CA250044/CA/NCI NIH HHS/United States
- T32 CA108462/CA/NCI NIH HHS/United States
- R01CA222508-01/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

