Intermittent hypoxia training enhances Aβ endocytosis by plaque associated microglia via VPS35-dependent TREM2 recycling in murine Alzheimer's disease
- PMID: 38831312
- PMCID: PMC11145795
- DOI: 10.1186/s13195-024-01489-6
Intermittent hypoxia training enhances Aβ endocytosis by plaque associated microglia via VPS35-dependent TREM2 recycling in murine Alzheimer's disease
Abstract
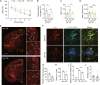
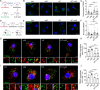
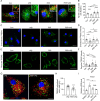
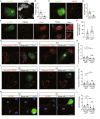
Background: Beta-amyloid (Aβ) deposition in the brain parenchyma is a crucial initiating step in the amyloid cascade hypothesis of Alzheimer's disease (AD) pathology. Furthermore, dysfunction of plaque-associated microglia, also known as disease-associated microglia (DAM) has been reported to accelerate Aβ deposition and cognitive impairment. Our previous research demonstrated that intermittent hypoxia training (IHT) improved AD pathology by upregulating autophagy in DAM, thereby enhancing oligomeric Aβ (oAβ) clearance. Considering that oAβ internalization is the initial stage of oAβ clearance, this study focused on the IHT mechanism involved in upregulating Aβ uptake by DAM.
Methods: IHT was administered to 8-month-old APP/PS1 mice or 6-month-old microglial vacuolar protein sorting 35 (VPS35) knockout mice in APP/PS1 background (MG VPS35 KO: APP/PS1) for 28 days. After the IHT, the spatial learning-memory capacity of the mice was assessed. Additionally, AD pathology was determined by estimating the nerve fiber and synapse density, Aβ plaque deposition, and Aβ load in the brain. A model of Aβ-exposed microglia was constructed and treated with IHT to explore the related mechanism. Finally, triggering receptor expressed on myeloid cells 2 (TREM2) intracellular recycling and Aβ internalization were measured using a fluorescence tracing technique.
Results: Our results showed that IHT ameliorated cognitive function and Aβ pathology. In particular, IHT enhanced Aβ endocytosis by augmenting the intracellular transport function of microglial TREM2, thereby contributing to Aβ clearance. Furthermore, IHT specifically upregulated VPS35 in DAM, the primary cause for the enhanced intracellular recycling of TREM2. IHT lost ameliorative effect on Aβ pathology in MG VPS35 KO: APP/PS1 mice brain. Lastly, the IHT mechanism of VPS35 upregulation in DAM was mediated by the transcriptional regulation of VPS35 by transcription factor EB (TFEB).
Conclusion: IHT enhances Aβ endocytosis in DAM by upregulating VPS35-dependent TREM2 recycling, thereby facilitating oAβ clearance and mitigation of Aβ pathology. Moreover, the transcriptional regulation of VPS35 by TFEB demonstrates a close link between endocytosis and autophagy in microglia. Our study further elucidates the IHT mechanism in improving AD pathology and provides evidence supporting the potential application of IHT as a complementary therapy for AD.
Keywords: Alzheimer’s disease; Beta-amyloid endocytosis; Plaque-associated microglia; TFEB; TREM2 recycling; VPS35.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Intermittent hypoxia therapy ameliorates beta-amyloid pathology via TFEB-mediated autophagy in murine Alzheimer's disease.J Neuroinflammation. 2023 Oct 20;20(1):240. doi: 10.1186/s12974-023-02931-6. J Neuroinflammation. 2023. PMID: 37864249 Free PMC article.
-
Microglial mTOR Activation Upregulates Trem2 and Enhances β-Amyloid Plaque Clearance in the 5XFAD Alzheimer's Disease Model.J Neurosci. 2022 Jul 6;42(27):5294-5313. doi: 10.1523/JNEUROSCI.2427-21.2022. Epub 2022 Jun 7. J Neurosci. 2022. PMID: 35672148 Free PMC article.
-
Vps35-dependent recycling of Trem2 regulates microglial function.Traffic. 2016 Dec;17(12):1286-1296. doi: 10.1111/tra.12451. Epub 2016 Nov 1. Traffic. 2016. PMID: 27717139
-
Untangling the Role of TREM2 in Conjugation with Microglia in Neuronal Dysfunction: A Hypothesis on a Novel Pathway in the Pathophysiology of Alzheimer's Disease.J Alzheimers Dis. 2023;94(s1):S319-S333. doi: 10.3233/JAD-221070. J Alzheimers Dis. 2023. PMID: 36683512 Free PMC article. Review.
-
TREM2 Function in Alzheimer's Disease and Neurodegeneration.ACS Chem Neurosci. 2016 Apr 20;7(4):420-7. doi: 10.1021/acschemneuro.5b00313. Epub 2016 Feb 19. ACS Chem Neurosci. 2016. PMID: 26854967 Review.
Cited by
-
Intermittent Hypoxia Induces Cognitive Dysfunction and Hippocampal Gene Expression Changes in a Mouse Model of Obstructive Sleep Apnea.Int J Mol Sci. 2025 Aug 3;26(15):7495. doi: 10.3390/ijms26157495. Int J Mol Sci. 2025. PMID: 40806623 Free PMC article.
-
VPS35-Retromer: Multifunctional Roles in Various Biological Processes - A Focus on Neurodegenerative Diseases and Cancer.J Inflamm Res. 2025 Apr 3;18:4665-4680. doi: 10.2147/JIR.S510768. eCollection 2025. J Inflamm Res. 2025. PMID: 40195959 Free PMC article. Review.
-
Astrocytes Lingering at a Crossroads: Neuroprotection and Neurodegeneration in Neurocognitive Dysfunction.Int J Biol Sci. 2025 Apr 28;21(7):3122-3143. doi: 10.7150/ijbs.109315. eCollection 2025. Int J Biol Sci. 2025. PMID: 40384856 Free PMC article. Review.
-
Pro-Inflammatory Microglia Exacerbate High-Altitude-Induced Cognitive Impairment by Driving Lipid Droplet Accumulation in Astrocytes.Antioxidants (Basel). 2025 Jul 26;14(8):918. doi: 10.3390/antiox14080918. Antioxidants (Basel). 2025. PMID: 40867817 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

