The neural and genetic underpinnings of different developmental trajectories of Attention-Deficit/Hyperactivity Symptoms in children and adolescents
- PMID: 38831366
- PMCID: PMC11149188
- DOI: 10.1186/s12916-024-03449-1
The neural and genetic underpinnings of different developmental trajectories of Attention-Deficit/Hyperactivity Symptoms in children and adolescents
Abstract
Background: The trajectory of attention-deficit hyperactivity disorder (ADHD) symptoms in children and adolescents, encompassing descending, stable, and ascending patterns, delineates their ADHD status as remission, persistence or late onset. However, the neural and genetic underpinnings governing the trajectory of ADHD remain inadequately elucidated.
Methods: In this study, we employed neuroimaging techniques, behavioral assessments, and genetic analyses on a cohort of 487 children aged 6-15 from the Children School Functions and Brain Development project at baseline and two follow-up tests for 1 year each (interval 1: 1.14 ± 0.32 years; interval 2: 1.14 ± 0.30 years). We applied a Latent class mixed model (LCMM) to identify the developmental trajectory of ADHD symptoms in children and adolescents, while investigating the neural correlates through gray matter volume (GMV) analysis and exploring the genetic underpinnings using polygenic risk scores (PRS).
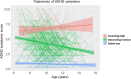
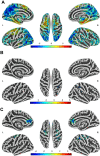
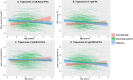
Results: This study identified three distinct trajectories (ascending-high, stable-low, and descending-medium) of ADHD symptoms from childhood through adolescence. Utilizing the linear mixed-effects (LME) model, we discovered that attention hub regions served as the neural basis for these three developmental trajectories. These regions encompassed the left anterior cingulate cortex/medial prefrontal cortex (ACC/mPFC), responsible for inhibitory control; the right inferior parietal lobule (IPL), which facilitated conscious focus on exogenous stimuli; and the bilateral middle frontal gyrus/precentral gyrus (MFG/PCG), accountable for regulating both dorsal and ventral attention networks while playing a crucial role in flexible modulation of endogenous and extrinsic attention. Furthermore, our findings revealed that individuals in the ascending-high group exhibited the highest PRS for ADHD, followed by those in the descending-medium group, with individuals in the stable-low group displaying the lowest PRS. Notably, both ascending-high and descending-medium groups had significantly higher PRS compared to the stable-low group.
Conclusions: The developmental trajectory of ADHD symptoms in the general population throughout childhood and adolescence can be reliably classified into ascending-high, stable-low, and descending-medium groups. The bilateral MFG/PCG, left ACC/mPFC, and right IPL may serve as crucial brain regions involved in attention processing, potentially determining these trajectories. Furthermore, the ascending-high pattern of ADHD symptoms exhibited the highest PRS for ADHD.
Keywords: ADHD; Children and adolescents; Genetic; Neurodevelopmental; Symptom trajectory.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Shared and Distinct Neurobiological Bases of Bipolar Disorder and Attention-Deficit/Hyperactivity Disorder in Children and Adolescents: A Comparative Meta-Analysis of Structural Abnormalities.J Am Acad Child Adolesc Psychiatry. 2024 Jun;63(6):586-604. doi: 10.1016/j.jaac.2023.09.551. Epub 2023 Dec 8. J Am Acad Child Adolesc Psychiatry. 2024. PMID: 38072245
-
Association of Genetic Risk Variants With Attention-Deficit/Hyperactivity Disorder Trajectories in the General Population.JAMA Psychiatry. 2016 Dec 1;73(12):1285-1292. doi: 10.1001/jamapsychiatry.2016.2817. JAMA Psychiatry. 2016. PMID: 27806167 Free PMC article.
-
Connections Between the Middle Frontal Gyrus and the Dorsoventral Attention Network Are Associated With the Development of Attentional Symptoms.Biol Psychiatry. 2025 Mar 1;97(5):531-539. doi: 10.1016/j.biopsych.2024.04.019. Epub 2024 May 6. Biol Psychiatry. 2025. PMID: 38718879
-
A multimodal neuroimaging meta-analysis of functional and structural brain abnormalities in attention-deficit/hyperactivity disorder.Prog Neuropsychopharmacol Biol Psychiatry. 2025 Jan 10;136:111199. doi: 10.1016/j.pnpbp.2024.111199. Epub 2024 Nov 29. Prog Neuropsychopharmacol Biol Psychiatry. 2025. PMID: 39615871
-
[Structural and functional neuroanatomy of attention-deficit hyperactivity disorder (ADHD)].Encephale. 2009 Apr;35(2):107-14. doi: 10.1016/j.encep.2008.01.005. Epub 2008 Jul 7. Encephale. 2009. PMID: 19393378 Review. French.
Cited by
-
Transcutaneous auricular vagus nerve stimulation as a potential therapy for attention deficit hyperactivity disorder: modulation of the noradrenergic pathway in the prefrontal lobe.Front Neurosci. 2024 Dec 4;18:1494272. doi: 10.3389/fnins.2024.1494272. eCollection 2024. Front Neurosci. 2024. PMID: 39697776 Free PMC article. Review.
-
Neural mechanisms of CALM intervention to improve CRCI in breast cancer survivors: an fMRI-based study.Am J Cancer Res. 2025 Apr 15;15(4):1733-1746. doi: 10.62347/OOVH5568. eCollection 2025. Am J Cancer Res. 2025. PMID: 40371132 Free PMC article.
-
Personality Traits in Adolescents with ADHD: Insights into Dimension Evaluation and Clinical Implications Using the Personality Inventory for the DSM-5 Questionnaire.J Clin Med. 2025 Apr 28;14(9):3048. doi: 10.3390/jcm14093048. J Clin Med. 2025. PMID: 40364080 Free PMC article.
-
Fluctuating course of attention-deficit/hyperactivity disorder across development: Multifactorial influences.World J Psychiatry. 2025 Aug 19;15(8):107780. doi: 10.5498/wjp.v15.i8.107780. eCollection 2025 Aug 19. World J Psychiatry. 2025. PMID: 40837790 Free PMC article. Review.
References
-
- American Psychiatric Association D, Association AP. Diagnostic and statistical manual of mental disorders: DSM-5, vol. 5. Washington DC: American Psychiatric Association; 2013.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

