Chrysanthemum morifolium Ramat extract and probiotics combination ameliorates metabolic disorders through regulating gut microbiota and PPARα subcellular localization
- PMID: 38831430
- PMCID: PMC11149226
- DOI: 10.1186/s13020-024-00950-w
Chrysanthemum morifolium Ramat extract and probiotics combination ameliorates metabolic disorders through regulating gut microbiota and PPARα subcellular localization
Abstract
Background: Chrysanthemum morifolium Ramat, a traditional Chinese medicine, has the effects on liver clearing, vision improving, and anti-inflammation. C. morifolium and probiotics have been individually studied for their beneficial effects on metabolic diseases. However, the underlying molecular mechanisms were not completely elucidated. This study aims to elucidate the potential molecular mechanisms of C. morifolium and probiotics combination (CP) on alleviating nonalcoholic fatty liver disease (NAFLD) and the dysregulation of glucose metabolism in high-fat diet (HFD)-fed mice.
Methods: The therapeutic effect of CP on metabolism was evaluated by liver histology and serum biochemical analysis, as well as glucose tolerance test. The impact of CP on gut microbiota was analyzed by 16S rRNA sequencing and fecal microbiota transplantation. Hepatic transcriptomic analysis was performed with the key genes and proteins validated by RT-qPCR and western blotting. In addition, whole body Pparα knockout (Pparα-/-) mice were used to confirm the CP-mediated pathway.
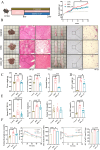
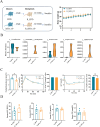
Results: CP supplementation ameliorated metabolic disorders by reducing body weight and hepatic steatosis, and improving glucose intolerance and insulin resistance in HFD fed mice. CP intervention mitigated the HFD-induced gut microbiota dysbiosis, which contributed at least in part, to the beneficial effect of improving glucose metabolism. In addition, hepatic transcriptomic analysis showed that CP modulated the expression of genes associated with lipid metabolism. CP downregulated the mRNA level of lipid droplet-binding proteins, such as Cidea and Cidec in the liver, leading to more substrates for fatty acid oxidation (FAO). Meanwhile, the expression of CPT1α, the rate-limiting enzyme of FAO, was significantly increased upon CP treatment. Mechanistically, though CP didn't affect the total PPARα level, it promoted the nuclear localization of PPARα, which contributed to the reduced expression of Cidea and Cidec, and increased expression of CPT1α, leading to activated FAO. Moreover, whole body PPARα deficiency abolished the anti-NAFLD effect of CP, suggesting the importance of PPARα in CP-mediated beneficial effect.
Conclusion: This study revealed the hypoglycemic and hepatoprotective effect of CP by regulating gut microbiota composition and PPARα subcellular localization, highlighting its potential for therapeutic candidate for metabolic disorders.
Keywords: Chrysanthemum morifolium Ramat; Gut microbiota; NAFLD; PPARα; Probiotics; T2DM.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that there are no known conflicts of interest associated with this publication.
Figures






Similar articles
-
Chrysanthemum morifolium attenuates metabolic and alcohol-associated liver disease via gut microbiota and PPARα/γ activation.Phytomedicine. 2024 Jul 25;130:155774. doi: 10.1016/j.phymed.2024.155774. Epub 2024 May 23. Phytomedicine. 2024. PMID: 38820659
-
Traditional Chinese Medicine formula Dai-Zong-Fang alleviating hepatic steatosis in db/db mice via gut microbiota modulation.Front Pharmacol. 2024 Jan 24;15:1337057. doi: 10.3389/fphar.2024.1337057. eCollection 2024. Front Pharmacol. 2024. PMID: 38327989 Free PMC article.
-
Lactobacillus plantarum FRT10 alleviated high-fat diet-induced obesity in mice through regulating the PPARα signal pathway and gut microbiota.Appl Microbiol Biotechnol. 2020 Jul;104(13):5959-5972. doi: 10.1007/s00253-020-10620-0. Epub 2020 May 14. Appl Microbiol Biotechnol. 2020. PMID: 32409945
-
Ban-xia-xie-xin-tang ameliorates hepatic steatosis by regulating Cidea and Cidec expression in HFD-fed mice.Phytomedicine. 2022 Oct;105:154351. doi: 10.1016/j.phymed.2022.154351. Epub 2022 Jul 21. Phytomedicine. 2022. PMID: 35908522
-
Research progress on pharmacological effects against liver and eye diseases of flavonoids present in Chrysanthum indicum L., Chrysanthemum morifolium Ramat., Buddleja officinalis Maxim. and Sophora japonica L.J Ethnopharmacol. 2025 Feb 10;338(Pt 2):119094. doi: 10.1016/j.jep.2024.119094. Epub 2024 Nov 10. J Ethnopharmacol. 2025. PMID: 39532220 Review.
Cited by
-
Gut microbiota in non-alcoholic fatty liver disease: Pathophysiology, diagnosis, and therapeutics.World J Hepatol. 2025 Jun 27;17(6):106849. doi: 10.4254/wjh.v17.i6.106849. World J Hepatol. 2025. PMID: 40606926 Free PMC article. Review.
-
Targeting lipid droplets and lipid droplet-associated proteins: a new perspective on natural compounds against metabolic diseases.Chin Med. 2024 Sep 4;19(1):120. doi: 10.1186/s13020-024-00988-w. Chin Med. 2024. PMID: 39232826 Free PMC article. Review.
-
Mentha haplocalyx Briq. (Mint): a comprehensive review on the botany, traditional uses, nutritional value, phytochemistry, health benefits, and applications.Chin Med. 2024 Dec 12;19(1):168. doi: 10.1186/s13020-024-01037-2. Chin Med. 2024. PMID: 39663516 Free PMC article. Review.
References
-
- Li Y, Lu Y, Nian M, Sheng Q, Zhang C, Han C, Dou X, Ding Y. Therapeutic potential and mechanism of Chinese herbal medicines in treating fibrotic liver disease. Chin J Nat Med. 2023;21(9):643–657. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

