Long non-coding RNA Small Nucleolar RNA Host Gene 4 ameliorates cigarette smoke-induced proliferation, apoptosis, inflammation, and airway remodeling in alveolar epithelial cells through the modulation of the mitogen-activated protein kinase signaling pathway via the microRNA-409-3p/Four and a Half LIM Domains 1 axis
- PMID: 38831471
- PMCID: PMC11149209
- DOI: 10.1186/s40001-024-01872-x
Long non-coding RNA Small Nucleolar RNA Host Gene 4 ameliorates cigarette smoke-induced proliferation, apoptosis, inflammation, and airway remodeling in alveolar epithelial cells through the modulation of the mitogen-activated protein kinase signaling pathway via the microRNA-409-3p/Four and a Half LIM Domains 1 axis
Abstract
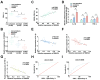
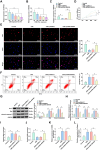
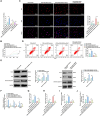
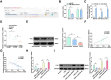
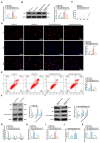
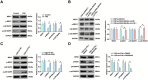
The long non-coding RNA (lncRNA) Small Nucleolar RNA Host Gene 4 (SNHG4) has been demonstrated to be significantly downregulated in various inflammatory conditions, yet its role in chronic obstructive pulmonary disease (COPD) remains elusive. This study aims to elucidate the biological function of SNHG4 in COPD and to unveil its potential molecular targets. Our findings reveal that both SNHG4 and Four and a Half LIM Domains 1 (FHL1) were markedly downregulated in COPD, whereas microRNA-409-3p (miR-409-3p) was upregulated. Importantly, SNHG4 exhibited a negative correlation with inflammatory markers in patients with COPD, but a positive correlation with forced expiratory volume in 1s percentage (FEV1%). SNHG4 distinguished COPD patients from non-smokers with high sensitivity, specificity, and accuracy. Overexpression of SNHG4 ameliorated cigarette smoke extract (CSE)-mediated inflammation, apoptosis, oxidative stress, and airway remodeling in 16HBE bronchial epithelial cells. These beneficial effects of SNHG4 overexpression were reversed by the overexpression of miR-409-3p or the silencing of FHL1. Mechanistically, SNHG4 competitively bound to miR-409-3p, mediating the expression of FHL1, and consequently improving inflammation, apoptosis, oxidative stress, and airway remodeling in 16HBE cells. Additionally, SNHG4 regulated the miR-409-3p/FHL1 axis to inhibit the activation of the mitogen-activated protein kinase (MAPK) pathway induced by CSE. In a murine model of COPD, knockdown of SNHG4 exacerbated CSE-induced pulmonary inflammation, apoptosis, and oxidative stress. In summary, our data affirm that SNHG4 mitigates pulmonary inflammation, apoptosis, and oxidative damage mediated by COPD through the regulation of the miR-409-3p/FHL1 axis.
Keywords: COPD; FHL1; Lnc SNHG4; MAPK; miR-409-3p.
© 2024. The Author(s).
Conflict of interest statement
Authors declared no conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

