Disturbance in cerebral blood microcirculation and hypoxic-ischemic microenvironment are associated with the development of brain metastasis
- PMID: 38831719
- PMCID: PMC11534324
- DOI: 10.1093/neuonc/noae094
Disturbance in cerebral blood microcirculation and hypoxic-ischemic microenvironment are associated with the development of brain metastasis
Abstract
Background: Brain metastases (BM) constitute an increasing challenge in oncology due to their impact on neurological function, limited treatment options, and poor prognosis. BM occurs through extravasation of circulating tumor cells across the blood-brain barrier. However, the extravasation processes are still poorly understood. We here propose a brain colonization process which mimics infarction-like microenvironmental reactions, that are dependent on Angiopoietin-2 (Ang-2) and vascular endothelial growth factor (VEGF).
Methods: In this study, intracardiac BM models were used, and cerebral blood microcirculation was monitored by 2-photon microscopy through a cranial window. BM formation was observed using cranial magnetic resonance, bioluminescent imaging, and postmortem autopsy. Ang-2/VEGF targeting strategies and Ang-2 gain-of-function (GOF) mice were employed to interfere with BM formation. In addition, vascular and stromal factors as well as clinical outcomes were analyzed in BM patients.
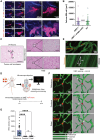
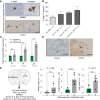
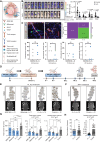
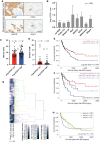
Results: Blood vessel occlusions by cancer cells were detected, accompanied by significant disturbances of cerebral blood microcirculation, and focal stroke-like histological signs. Cerebral endothelial cells showed an elevated Ang-2 expression both in mouse and human BM. Ang-2 GOF resulted in an increased BM burden. Combined anti-Ang-2/anti-VEGF therapy led to a decrease in brain metastasis size and number. Ang-2 expression in tumor vessels of established human BM negatively correlated with survival.
Conclusions: Our observations revealed a relationship between disturbance of cerebral blood microcirculation and brain metastasis formation. This suggests that vessel occlusion by tumor cells facilitates brain metastatic extravasation and seeding, while combined inhibition of microenvironmental effects of Ang-2 and VEGF prevents the outgrowth of macrometastases.
Keywords: VEGF; angiopoietin-2; anti-angiogenic therapy; brain metastases; perivascular niche.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society for Neuro-Oncology.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Comment in
-
Thrombosis, brain metastasis formation, and the perivascular metastatic niche-Novel insights from the tumor microvascular circulation.Neuro Oncol. 2024 Nov 4;26(11):2100-2101. doi: 10.1093/neuonc/noae168. Neuro Oncol. 2024. PMID: 39207022 Free PMC article. No abstract available.
References
-
- Nayak L, Lee EQ, Wen PY.. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14(1):48–54. - PubMed
-
- Achrol AS, Rennert RC, Anders C, et al. Brain metastases. Nat Rev Dis Prim. 2019;5(1):5. - PubMed
-
- Winkler F. The brain metastatic niche. J Molecular Med. 2015;93(11):1213–1220. - PubMed
-
- Avraham HK, Jiang S, Fu Y, et al. Angiopoietin-2 mediates blood-brain barrier impairment and colonization of triple-negative breast cancer cells in brain. J Pathol. 2014;232(3):369–381. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

