Rapidly repeated visual stimulation induces long-term potentiation of VEPs and increased content of membrane AMPA and NMDA receptors in the V1 cortex of cats
- PMID: 38831757
- PMCID: PMC11144871
- DOI: 10.3389/fnins.2024.1386801
Rapidly repeated visual stimulation induces long-term potentiation of VEPs and increased content of membrane AMPA and NMDA receptors in the V1 cortex of cats
Abstract
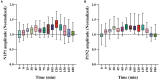
Studies report that rapidly repeated sensory stimulation can evoke LTP-like improvement of neural response in the sensory cortex. Whether this neural response potentiation is similar to the classic LTP induced by presynaptic electrical stimulation remains unclear. This study examined the effects of repeated high-frequency (9 Hz) versus low-frequency (1 Hz) visual stimulation on visually-evoked field potentials (VEPs) and the membrane protein content of AMPA / NMDA receptors in the primary visual cortex (V1) of cats. The results showed that repeated high-frequency visual stimulation (HFS) caused a long-term improvement in peak-to-peak amplitude of V1-cortical VEPs in response to visual stimuli at HFS-stimulated orientation (SO: 90°) and non-stimulated orientation (NSO: 180°), but the effect exhibited variations depending on stimulus orientation: the amplitude increase of VEPs in response to visual stimuli at SO was larger, reached a maximum earlier and lasted longer than at NSO. By contrast, repeated low-frequency visual stimulation (LFS) had not significantly affected the amplitude of V1-cortical VEPs in response to visual stimuli at both SO and NSO. Furthermore, the membrane protein content of the key subunit GluA1 of AMPA receptors and main subunit NR1 of AMPA receptors in V1 cortex was significantly increased after HFS but not LFS when compared with that of control cats. Taken together, these results indicate that HFS can induce LTP-like improvement of VEPs and an increase in membrane protein of AMPA and NMDA receptors in the V1 cortex of cats, which is similar to but less specific to stimulus orientation than the classic LTP.
Keywords: AMPA and NMDA receptors; cat; long-term potentiation; primary visual cortex; repeated high-frequency visual stimulation; visually-evoked field potentials.
Copyright © 2024 Chen, Lu, Cheng, Ye and Hua.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Agboada D., Mosayebi-Samani M., Kuo M. F., Nitsche M. A. (2020). Induction of long-term potentiation-like plasticity in the primary motor cortex with repeated anodal transcranial direct current stimulation - better effects with intensified protocols? Brain Stimul. 13, 987–997. doi: 10.1016/j.brs.2020.04.009, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

