Red blood cell flickering activity locally controlled by holographic optical tweezers
- PMID: 38832008
- PMCID: PMC11145342
- DOI: 10.1016/j.isci.2024.109915
Red blood cell flickering activity locally controlled by holographic optical tweezers
Abstract
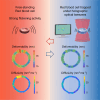
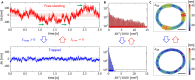
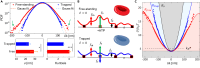
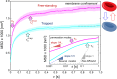
Red blood cells possess a singular mechanobiology, enabling efficient navigation through capillaries smaller than their own size. Their plasma membrane exhibits non-equilibrium shape fluctuation, often reported as enhanced flickering activity. Such active membrane motion is propelled by motor proteins that mediate interactions between the spectrin skeleton and the lipid bilayer. However, modulating the flickering in living red blood cells without permanently altering their mechanical properties represents a significant challenge. In this study, we developed holographic optical tweezers to generate a force field distributed along the equatorial membrane contour of individual red blood cells. In free-standing red blood cells, we observed heterogeneous flickering activity, attributed to localized membrane kickers. By employing holographic optical forces, these active kickers can be selectively halted under minimal invasion. Our findings shed light on the dynamics of membrane flickering and established a manipulation tool that could open new avenues for investigating mechanotransduction processes in living cells.
Keywords: Natural sciences; Optical imaging.
© 2024 The Authors.
Conflict of interest statement
The authors declare no competing interest.
Figures

Similar articles
-
How should the optical tweezers experiment be used to characterize the red blood cell membrane mechanics?Biomech Model Mechanobiol. 2017 Oct;16(5):1645-1657. doi: 10.1007/s10237-017-0910-x. Epub 2017 May 3. Biomech Model Mechanobiol. 2017. PMID: 28470421
-
Deformation behaviour of stomatocyte, discocyte and echinocyte red blood cell morphologies during optical tweezers stretching.Biomech Model Mechanobiol. 2020 Oct;19(5):1827-1843. doi: 10.1007/s10237-020-01311-w. Epub 2020 Feb 25. Biomech Model Mechanobiol. 2020. PMID: 32100179
-
Correlated flickering of erythrocytes membrane observed with dual time resolved membrane fluctuation spectroscopy under different D-glucose concentrations.Sci Rep. 2021 Jan 28;11(1):2429. doi: 10.1038/s41598-021-82018-5. Sci Rep. 2021. PMID: 33510337 Free PMC article.
-
Shining Light in Mechanobiology: Optical Tweezers, Scissors, and Beyond.ACS Photonics. 2024 Mar 11;11(3):917-940. doi: 10.1021/acsphotonics.4c00064. eCollection 2024 Mar 20. ACS Photonics. 2024. PMID: 38523746 Free PMC article. Review.
-
Optothermal Manipulations of Colloidal Particles and Living Cells.Acc Chem Res. 2018 Jun 19;51(6):1465-1474. doi: 10.1021/acs.accounts.8b00102. Epub 2018 May 25. Acc Chem Res. 2018. PMID: 29799720 Free PMC article. Review.
Cited by
-
Mechanical adaptivity of red blood cell flickering to extrinsic membrane stiffening by the solid-like biosurfactant β-Aescin.Biophys J. 2025 May 6;124(9):1478-1495. doi: 10.1016/j.bpj.2025.03.027. Epub 2025 Apr 1. Biophys J. 2025. PMID: 40176347 Free PMC article.
References
-
- Orapiriyakul W., Tsimbouri M.P., Childs P., Campsie P., Wells J., Fernandez-Yague M.A., Burgess K., Tanner K.E., Tassieri M., Meek D., et al. Nanovibrational Stimulation of Mesenchymal Stem Cells Induces Therapeutic Reactive Oxygen Species and Inflammation for Three-Dimensional Bone Tissue Engineering. ACS Nano. 2020;14:10027–10044. doi: 10.1021/acsnano.0c03130. - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources

