Nrf2 pathway activation promotes the expression of genes related to glutathione metabolism in alcohol-exposed astrocytes
- PMID: 38832034
- PMCID: PMC11146317
- DOI: 10.7717/peerj.17541
Nrf2 pathway activation promotes the expression of genes related to glutathione metabolism in alcohol-exposed astrocytes
Abstract
Introduction: Oxidative and antioxidant pathways play essential roles in the development of alcohol-induced brain injury. The Nrf2 pathway is an endogenous antioxidant response pathway, but there has been little research on the role of Nrf2 in alcohol-related diseases. Thus, we examined the effects of alcohol and an Nrf2 agonist (TBHQ) on astrocyte function, mRNA expression, and metabolite content to further explore the protective mechanisms of Nrf2 agonists in astrocytes following alcohol exposure.
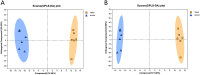
Methods: CTX TNA2 astrocytes were cultured with alcohol and TBHQ and then subjected to transcriptome sequencing, LC-MS/MS analysis, quantitative reverse transcription polymerase chain reaction (qRT-PCR), and malondialdehyde (MDA) and superoxide dismutase (SOD) activity assays.
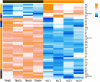
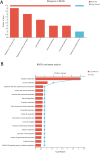
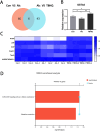
Results: Alcohol exposure significantly increased malondialdehyde (MDA) levels while decreasing superoxide dismutase (SOD) levels in astrocytes. Treatment with TBHQ effectively reversed these effects, demonstrating its protective role against oxidative stress induced by alcohol. Transcriptome sequencing and qRT-PCR analysis revealed that TBHQ specifically upregulates genes involved in glutathione metabolism, including a notable increase in the expression of the glutathione S-transferase A5 (GSTA5) gene, which was suppressed by alcohol exposure. Additionally, metabolomic analysis showed that TBHQ regulates key components of ether lipid metabolism in alcohol-exposed astrocytes, with significant reductions in the levels of lysophosphatidylcholine (18:0) (LysoPC (18:0)) and 2-acetyl-1-alkyl-sn-glycero-3-phosphocholine, both of which are critical markers in the ether lipid metabolic pathway.
Discussion: The findings underscore the role of TBHQ as an Nrf2 agonist in mitigating alcohol-induced oxidative damage in astrocytes by modulating glutathione metabolism and ether lipid metabolism. The regulation of GSTA5 gene expression emerges as a key mechanism through which Nrf2 agonists confer neuroprotection against oxidative stress and lipid oxidation. These insights pave the way for potential therapeutic strategies targeting the Nrf2 pathway to protect astrocytes from alcohol-induced damage.
Keywords: Alcohol; Astrocyte; LC-MS/MS; Nrf2 pathway; Transcriptome sequencing.
© 2024 Li et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Activation of Nrf2/ARE signaling pathway attenuates lanthanum chloride induced injuries in primary rat astrocytes.Metallomics. 2017 Aug 16;9(8):1120-1131. doi: 10.1039/c7mt00182g. Metallomics. 2017. PMID: 28722058
-
Tert-Butylhydroquinone Protects Liver Against Ischemia/Reperfusion Injury in Rats Through Nrf2-Activating Anti-Oxidative Activity.Transplant Proc. 2017 Mar;49(2):366-372. doi: 10.1016/j.transproceed.2016.12.008. Transplant Proc. 2017. PMID: 28219600
-
Prolactin is an Endogenous Antioxidant Factor in Astrocytes That Limits Oxidative Stress-Induced Astrocytic Cell Death via the STAT3/NRF2 Signaling Pathway.Neurochem Res. 2024 Jul;49(7):1879-1901. doi: 10.1007/s11064-024-04147-3. Epub 2024 May 17. Neurochem Res. 2024. PMID: 38755517 Free PMC article.
-
The Nrf2-ARE pathway: an indicator and modulator of oxidative stress in neurodegeneration.Ann N Y Acad Sci. 2008 Dec;1147:61-9. doi: 10.1196/annals.1427.036. Ann N Y Acad Sci. 2008. PMID: 19076431 Free PMC article. Review.
-
Adaptive regulation of the brain's antioxidant defences by neurons and astrocytes.Free Radic Biol Med. 2016 Nov;100:147-152. doi: 10.1016/j.freeradbiomed.2016.06.027. Epub 2016 Jun 27. Free Radic Biol Med. 2016. PMID: 27365123 Free PMC article. Review.
References
-
- Barcia JM, Flores-Bellver M, Muriach M, Sancho-Pelluz J, Lopez-Malo D, Urdaneta AC, Martinez-Gil N, Atienzar-Aroca S, Romero FJ. Matching diabetes and alcoholism: oxidative stress, inflammation, and neurogenesis are commonly involved. Mediators of Inflammation. 2015;2015(4):624287. doi: 10.1155/2015/624287. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

