MELD3.0 is superior to MELDNa and MELD for prediction of mortality in patients with cirrhosis: An external validation in a multi-ethnic population
- PMID: 38832135
- PMCID: PMC11144281
- DOI: 10.1002/jgh3.13098
MELD3.0 is superior to MELDNa and MELD for prediction of mortality in patients with cirrhosis: An external validation in a multi-ethnic population
Abstract
Background and aim: The model for end-stage liver disease (MELD) was updated to MELDNa and recently to MELD3.0 to predict survival of cirrhotic patients. We validated the prognostic performance of MELD3.0 and compared with MELDNa and MELD amongst cirrhotic inpatients.
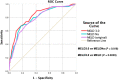
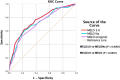
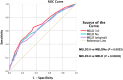
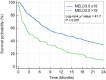
Methods: Demographical, clinical, biochemical, and survival data of cirrhotic inpatients in Singapore General Hospital (SGH) from 01 January 2018 to 31 December 2018, were studied retrospectively. Patients were followed up from first admission in 2018 until death or until 01 April 2023. Area under the receiver operating characteristic curves (AUROC) were computed for the discriminative effects of MELD3.0, MELDNa, and MELD to predict 30-, 90-, and 365-day mortalities. AUROC was compared with DeLong's test. The cutoff MELD3.0 score for patients at high risk of 30-day mortality was determined using Youden's Index. Survival curves of patients with MELD3.0 score above and below the cutoff were estimated with Kaplan-Meier method and compared with log-rank analysis.
Results: Totally 862 patients were included (median age 71.0 years [interquartile range, IQR: 64.0-79.0], 65.4% males, 75.8% Chinese). Proportion of patients with Child-Turcotte-Pugh classes A/B/C were 55.5%/35.5%/9.0%. Median MELD3.0/MELDNa/MELD scores were 12.2 (IQR: 8.7-18.3)/11.0 (IQR: 8.0-17.5)/10.3 (IQR: 7.8-15.0). Median time of follow-up was 51.9 months (IQR: 8.5-59.6). The proportion of 30-/90-/365-day mortalities was 5.7%/13.2%/26.9%. AUROC of MELD3.0/MELDNa/MELD in predicting 30-, 90-, and 365-day mortalities, respectively, were 0.823/0.793/0.783, 0.754/0.724/0.707, 0.682/0.654/0.644 (P < 0.05). Optimal cutoff to predict 30-day mortality was MELD3.0 > 19 (sensitivity = 67.4%, specificity = 82.4%). Patients with MELD3.0 > 19, compared with patients with MELD3.0 ≤ 19, had shorter median time to death (98.0 days [IQR: 28.8-398.0] vs 390.0 days [IQR: 134.3-927.5]), and higher proportion of 30-day mortality (68.8% vs 43.0%) (P < 0.001).
Conclusion: MELD3.0 performs better than MELDNa and MELD in predicting mortality in cirrhotic inpatients. MELD3.0 > 19 predicts higher 30-day mortality.
Keywords: liver cirrhosis; liver failure; liver transplantation; model for end‐stage liver disease.
© 2024 The Author(s). JGH Open published by Journal of Gastroenterology and Hepatology Foundation and John Wiley & Sons Australia, Ltd.
Figures
Similar articles
-
Development and Validation of a Scoring System That Includes Corrected QT Interval for Risk Analysis of Patients With Cirrhosis and Gastrointestinal Bleeding.Clin Gastroenterol Hepatol. 2019 Jun;17(7):1388-1397.e1. doi: 10.1016/j.cgh.2018.12.006. Epub 2018 Dec 15. Clin Gastroenterol Hepatol. 2019. PMID: 30557740
-
The Refit model for end-stage liver disease-Na is not a better predictor of mortality than the Refit model for end-stage liver disease in patients with cirrhosis and ascites.Clin Mol Hepatol. 2014 Mar;20(1):47-55. doi: 10.3350/cmh.2014.20.1.47. Epub 2014 Mar 26. Clin Mol Hepatol. 2014. PMID: 24757658 Free PMC article.
-
Different models in predicting the short-term prognosis of patients with hepatitis B virus-related acute-on-chronic liver failure.Ann Hepatol. 2012 May-Jun;11(3):311-9. Ann Hepatol. 2012. PMID: 22481448
-
Assessing liver dysfunction in cirrhosis: role of the model for end-stage liver disease and its derived systems.J Chin Med Assoc. 2013 Aug;76(8):419-24. doi: 10.1016/j.jcma.2013.04.010. Epub 2013 Jun 5. J Chin Med Assoc. 2013. PMID: 23746532 Review.
-
Ability of King's College Criteria and Model for End-Stage Liver Disease Scores to Predict Mortality of Patients With Acute Liver Failure: A Meta-analysis.Clin Gastroenterol Hepatol. 2016 Apr;14(4):516-525.e5; quiz e43-e45. doi: 10.1016/j.cgh.2015.10.007. Epub 2015 Oct 20. Clin Gastroenterol Hepatol. 2016. PMID: 26499930 Review.
Cited by
-
Validation of ICD-10 Consensus Code Set for Cirrhosis Detection Using Electronic Health Records in an Asian Population.JGH Open. 2025 May 6;9(5):e70156. doi: 10.1002/jgh3.70156. eCollection 2025 May. JGH Open. 2025. PMID: 40330254 Free PMC article.
-
Preliminary experience of combined dual plasma molecular adsorption system and plasma exchange in pediatric acute liver failure: a retrospective case series.BMC Pediatr. 2025 Mar 3;25(1):163. doi: 10.1186/s12887-025-05520-z. BMC Pediatr. 2025. PMID: 40033216 Free PMC article.
-
The unwell patient with advanced chronic liver disease: when to use each score?BMC Med. 2025 Jul 9;23(1):413. doi: 10.1186/s12916-025-04185-w. BMC Med. 2025. PMID: 40629399 Free PMC article. Review.
References
-
- Kamath PS, Wiesner RH, Malinchoc M et al. A model to predict survival in patients with end‐stage liver disease. Hepatology. 2001; 33: 464–470. - PubMed
-
- Said A, Williams J, Holden J et al. Model for end stage liver disease score predicts mortality across a broad spectrum of liver disease. J. Hepatol. 2004; 40: 897–903. - PubMed
-
- Wiesner R, Edwards E, Freeman R et al. Model for end‐stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003; 124: 91–96. - PubMed
-
- Neuberger J. Allocation of donor livers? Is MELD enough? Liver Transpl. 2004; 10: 908–910. - PubMed
-
- Freeman RB. MELD: the holy grail of organ allocation? J. Hepatol. 2005; 42: 16–20. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

