A bacterial sense of touch: T4P retraction motor as a means of surface sensing by Pseudomonas aeruginosa PA14
- PMID: 38832786
- PMCID: PMC11270903
- DOI: 10.1128/jb.00442-23
A bacterial sense of touch: T4P retraction motor as a means of surface sensing by Pseudomonas aeruginosa PA14
Abstract
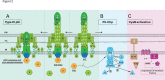
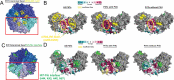
Most microbial cells found in nature exist in matrix-covered, surface-attached communities known as biofilms. This mode of growth is initiated by the ability of the microbe to sense a surface on which to grow. The opportunistic pathogen Pseudomonas aeruginosa (Pa) PA14 utilizes a single polar flagellum and type 4 pili (T4P) to sense surfaces. For Pa, T4P-dependent "twitching" motility is characterized by effectively pulling the cell across a surface through a complex process of cooperative binding, pulling, and unbinding. T4P retraction is powered by hexameric ATPases. Pa cells that have engaged a surface increase production of the second messenger cyclic AMP (cAMP) over multiple generations via the Pil-Chp system. This rise in cAMP allows cells and their progeny to become better adapted for surface attachment and activates virulence pathways through the cAMP-binding transcription factor Vfr. While many studies have focused on mechanisms of T4P twitching and regulation of T4P production and function by the Pil-Chp system, the mechanism by which Pa senses and relays a surface-engagement signal to the cell is still an open question. Here we review the current state of the surface sensing literature for Pa, with a focus on T4P, and propose an integrated model of surface sensing whereby the retraction motor PilT senses and relays the signal to the Pil-Chp system via PilJ to drive cAMP production and adaptation to a surface lifestyle.
Keywords: Pil-Chp; PilT; Pseudomonas aeruginosa; biofilm; motor; surface sensing; type 4 pili.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

