Candida albicans and Candida glabrata: global priority pathogens
- PMID: 38832801
- PMCID: PMC11332356
- DOI: 10.1128/mmbr.00021-23
Candida albicans and Candida glabrata: global priority pathogens
Abstract
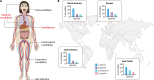
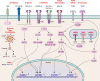
SUMMARYA significant increase in the incidence of Candida-mediated infections has been observed in the last decade, mainly due to rising numbers of susceptible individuals. Recently, the World Health Organization published its first fungal pathogen priority list, with Candida species listed in medium, high, and critical priority categories. This review is a synthesis of information and recent advances in our understanding of two of these species-Candida albicans and Candida glabrata. Of these, C. albicans is the most common cause of candidemia around the world and is categorized as a critical priority pathogen. C. glabrata is considered a high-priority pathogen and has become an increasingly important cause of candidemia in recent years. It is now the second most common causative agent of candidemia in many geographical regions. Despite their differences and phylogenetic divergence, they are successful as pathogens and commensals of humans. Both species can cause a broad variety of infections, ranging from superficial to potentially lethal systemic infections. While they share similarities in certain infection strategies, including tissue adhesion and invasion, they differ significantly in key aspects of their biology, interaction with immune cells, host damage strategies, and metabolic adaptations. Here we provide insights on key aspects of their biology, epidemiology, commensal and pathogenic lifestyles, interactions with the immune system, and antifungal resistance.
Keywords: Candida albicans; Candida glabrata; Nakaseomyces glabratus; WHO fungal priority list; antifungal resistance; commensalism; diagnostics; epidemiology; host response; pathogenicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Organization WH . 2022. WHO fungal priority pathogens list to guide research, development and public health action. Available from: https://www.who.int/publications/i/item/9789240060241
Publication types
MeSH terms
Substances
Grants and funding
- 13N15714/Bundesministerium für Bildung und Forschung (BMBF)
- MR/N006364/2/Medical Research Council Centre for Medical Mycology (MRC CMM)
- University of Exeter
- 101873, 200208, 215599/Wellcome Trust (WT)
- MR/M026663/2/UKRI | Medical Research Council (MRC)
- 224323/WT_/Wellcome Trust/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- SAS-2015-HKI-LWC/Bundesministerium für Bildung und Forschung (BMBF)
- National Institute for Health and Care Research Exeter Biomedical Research Centre
- 528/20-1/Deutsche Forschungsgemeinschaft (DFG)
- 467 390713860/Deutsche Forschungsgemeinschaft (DFG)
LinkOut - more resources
Full Text Sources
Medical

