Effectiveness of the flash glucose monitoring system in preventing severe hypoglycemic episodes and in improving glucose metrics and quality of life in subjects with type 1 diabetes at high risk of acute diabetes complications
- PMID: 38833007
- PMCID: PMC11379770
- DOI: 10.1007/s00592-024-02298-x
Effectiveness of the flash glucose monitoring system in preventing severe hypoglycemic episodes and in improving glucose metrics and quality of life in subjects with type 1 diabetes at high risk of acute diabetes complications
Abstract
Aims: To assess the effectiveness of the intermittent-scanned continuous glucose monitoring (isCGM) system in preventing severe hypoglycemic episodes and in improving glucose parameters and quality of life.
Methods: Four hundred T1D individuals were enrolled in a prospective real-word study with an intermittently scanned continuous glucose monitoring device during the 12-months follow-up. The primary endpoint was the incidence of severe hypoglycemic events.
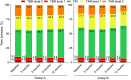
Results: 82% of subjects were naïve to the use of the device (group A) and 18% were already wearing the system (group B). The cumulative incidence of severe hypoglycemia (SH) at 12 months was 12.06 per 100 person-year (95% CI: 8.35-16.85) in group A and 10.14 (95% CI: 4.08-20.90) in group B without inter-group differences. In group A there was a significant decrease in SH at 12 months compared to 3 months period (p = 0.005). Time in glucose range significantly increased in both groups accompanied with a significant decrease in glucose variability. HbA1c showed a progressive significant time-dependent decrease in group A. The use of the device significantly improved the perceived quality of life.
Conclusion: This study confirmed the effectiveness of the isCGM in reducing hypoglycemic risk without glucose deterioration, with potential benefits on adverse outcomes in T1D individuals.
Trial registration: ClinicalTrials.gov registration no. NCT04060732.
Keywords: Intermittent-scanned continuous blood glucose monitoring; Severe hypoglycemia; Type 1 diabetes.
© 2024. The Author(s).
Conflict of interest statement
ADC has received lecture fees from MSD, AstraZeneca, Eli Lilly, Sanofi, DOC generici, Servier. PDB has been a board member/advisory panel for Abbott Italia, Astra Zeneca Italia, Allergan/Abvie, Bayer Italia, Boehringer Italia, Eli Lilly Italia, Glaxo Italia, Menarini Diagnostic, Novonordisk Italia, Sanofi Italia and speaker bureau for Abbott Italia, Astra Zeneca Italia, Allergan/Abbvie, Ascensia Italia, Bayer Italia, Boehringer Italia, Eli Lilly Italia, Guidotti, Insulet, Menarini Diagnostic, Novonordisk Italia, Sanofi Italia, Theras Italia. RCB has received lecture fees from AstraZeneca, Eli Lilly, Sanofi, MSD, Janssen and has been a board member/advisory panel for Eli Lilly, Sanofi, MSD, Amgen. RA, GB, DR, AS, GM, AVC, VM, MB, MM, AV, MCC, APS, MA, FF have no potential conflicts of interest. No other potential conflicts of interest relevant to this article were reported.
Figures


References
-
- Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C, (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329:977–986. 10.1056/NEJM199309303291401 10.1056/NEJM199309303291401 - DOI - PubMed
-
- Williams SA, Pollack MF, Dibonaventura M (2011) Effects of hypoglycemia on health-related quality of life, treatment satisfaction and healthcare resource utilization in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 91(3):363–370. 10.1016/j.diabres.2010.12.027 10.1016/j.diabres.2010.12.027 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

