Final analyses of the prospective controlled trial on the efficacy of uracil and tegafur/leucovorin as an adjuvant treatment for stage II colon cancer with risk factors for recurrence using propensity score-based methods (JFMC46-1201)
- PMID: 38833114
- PMCID: PMC11347494
- DOI: 10.1007/s10147-024-02565-5
Final analyses of the prospective controlled trial on the efficacy of uracil and tegafur/leucovorin as an adjuvant treatment for stage II colon cancer with risk factors for recurrence using propensity score-based methods (JFMC46-1201)
Abstract
Background: The efficacy of adjuvant chemotherapy for high-risk stage II colon cancer (CC) has not been well established. Using propensity score matching, we previously reported that the 3-year disease-free survival (DFS) rate was significantly higher in patients treated with uracil and tegafur plus leucovorin (UFT/LV) against surgery alone. We report the final results, including updated 5-year overall survival (OS) rates and risk factor analysis outcomes.
Methods: In total, 1902 high-risk stage II CC patients with T4, perforation/penetration, poorly differentiated adenocarcinoma/mucinous carcinoma, and/or < 12 dissected lymph nodes were enrolled in this prospective, non-randomized controlled study based on their self-selected treatment. Oral UFT/LV therapy was administered for six months after surgery.
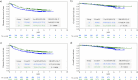
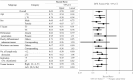
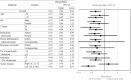
Results: Of the 1880 eligible patients, 402 in Group A (surgery alone) and 804 in Group B (UFT/LV) were propensity score-matched. The 5-year DFS rate was significantly higher in Group B than in Group A (P = 0.0008). The 5-year OS rates were not significantly different between groups. The inverse probability of treatment weighting revealed significantly higher 5-year DFS (P = 0.0006) and 5-year OS (P = 0.0122) rates in group B than in group A. Multivariate analyses revealed that male sex, age ≥ 70 years, T4, < 12 dissected lymph nodes, and no adjuvant chemotherapy were significant risk factors for DFS and/or OS.
Conclusion: The follow-up data from our prospective non-randomized controlled study revealed a considerable survival advantage in DFS offered by adjuvant chemotherapy with UFT/LV administered for six months over surgery alone in individuals with high-risk stage II CC.
Trial registration: Japan Registry of Clinical Trials: jRCTs031180155 (date of registration: 25/02/2019), UMIN Clinical Trials Registry: UMIN000007783 (date of registration: 18/04/2012).
Keywords: Adjuvant chemotherapy; Colon cancer; High-risk stage II; Inverse probability of treatment-weighting; Leucovorin; Propensity score; Risk factor; Tegafur; Uracil.
© 2024. The Author(s).
Conflict of interest statement
The authors declare the following financial interests/personal relationships, which may be considered potential competing interests: Takao Takahashi was awarded an endowed chair from Yakult Honsha outside the submitted work. Outside the submitted work, Hideo Baba received a grant and personal fees from Taiho Pharmaceutical. Outside the submitted work, Ichinosuke Hyodo received grants and personal fees from Taiho Pharmaceutical, Asahi Kasei Pharma, Chugai Pharmaceutical, Eisai, and Ono Pharmaceutical. Satoshi Morita received personal fees outside the submitted work from Bristol-Myers Squibb, Chugai Pharmaceutical, Taiho Pharmaceutical, Eli Lilly Japan, and AstraZeneca. All other authors have no conflicts of interest to declare.
Figures
References
-
- National comprehensive cancer network. (2014) Clinical practice guidelines in oncology (NCCN Guidelines®). Colon Cancer. Version 2.2015 http://www2.tri-kobe.org/nccn/guideline/archive/colorectal2015/english/c...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

