Treatment of Locally Advanced Rectal Cancer in the Era of Total Neoadjuvant Therapy: A Systematic Review and Network Meta-Analysis
- PMID: 38833249
- PMCID: PMC11151159
- DOI: 10.1001/jamanetworkopen.2024.14702
Treatment of Locally Advanced Rectal Cancer in the Era of Total Neoadjuvant Therapy: A Systematic Review and Network Meta-Analysis
Abstract
Importance: Treatment of locally advanced rectal cancer (LARC) involves neoadjuvant chemoradiotherapy plus total mesorectal excision and adjuvant chemotherapy. However, total neoadjuvant therapy (TNT) protocols (ie, preoperative chemotherapy in addition to radiotherapy) may allow better adherence and early treatment of distant micrometastases and may increase pathological complete response (pCR) rates.
Objective: To assess the efficacy and tolerability of TNT protocols for LARC.
Data sources: MEDLINE, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), and Web of Science Core Collection electronic databases and ClinicalTrials.gov for unpublished studies were searched from inception to March 2, 2024.
Study selection: Randomized clinical trials including adults with LARC who underwent rectal resection as a final treatment were included. Studies including nonoperative treatment (watch-and-wait strategy), treatments other than rectal resection, immunotherapy, or antiangiogenic agents were excluded. Among the initially identified studies, 2.9% met the selection criteria.
Data extraction and synthesis: Two authors independently screened the records and extracted data. Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)-compliant pairwise and network meta-analyses with a random-effects model were performed in a frequentist framework, and the certainty of evidence was assessed according to the confidence in network meta-analysis approach.
Main outcomes and measures: The primary outcome was pCR, defined as the absence of residual tumor at pathological assessment after surgery. Secondary outcomes included tolerability, toxic effects, perioperative outcomes, and long-term survival.
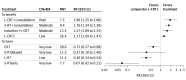
Results: Of 925 records identified, 27 randomized clinical trials, including 13 413 adults aged 18 years or older (median age, 60.0 years [range, 42.0-63.5 years]; 67.2% male) contributed to the primary network meta-analysis. With regard to pCR, long-course chemoradiotherapy (L-CRT) plus consolidation chemotherapy (relative risk [RR], 1.96; 95% CI, 1.25-3.06), short-course radiotherapy (S-RT) plus consolidation chemotherapy (RR, 1.76; 95% CI, 1.34-2.30), and induction chemotherapy plus L-CRT (RR, 1.57; 95% CI, 1.09-2.25) outperformed standard L-CRT with single-agent fluoropyrimidine-based chemotherapy. Considering 3-year disease-free survival, S-RT plus consolidation chemotherapy (RR, 1.08; 95% CI, 1.01-1.14) and induction chemotherapy plus L-CRT (RR, 1.12; 95% CI, 1.01-1.24) outperformed L-CRT, in spite of an increased 5-year locoregional recurrence rate of S-RT plus consolidation chemotherapy (RR, 1.65; 95% CI, 1.03-2.63).
Conclusions and relevance: In this systematic review and network meta-analysis, 3 TNT protocols were identified to outperform the current standard of care in terms of pCR rates, with good tolerability and optimal postoperative outcomes, suggesting they should be recognized as first-line treatments.
Conflict of interest statement
Figures


References
-
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): rectal cancer. Version 3.2022. October 27, 2022. Accessed November 20, 2022. https://www.nccn.org/guidelines/category_1
-
- You YN, Hardiman KM, Bafford A, et al. ; Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons . The American Society of Colon and Rectal Surgeons clinical practice guidelines for the management of rectal cancer. Dis Colon Rectum. 2020;63(9):1191-1222. doi: 10.1097/DCR.0000000000001762 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

