A Neuron-Mast Cell Axis Regulates Skin Microcirculation in Diabetes
- PMID: 38833271
- PMCID: PMC11573700
- DOI: 10.2337/db23-0862
A Neuron-Mast Cell Axis Regulates Skin Microcirculation in Diabetes
Abstract
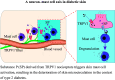
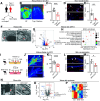
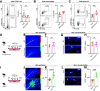
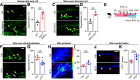
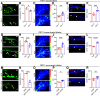
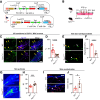
Changes in microcirculation lead to the progression of organ pathology in diabetes. Although neuroimmune interactions contribute to a variety of conditions, it is still unclear whether abnormal neural activities affect microcirculation related to diabetes. Using laser speckle contrast imaging, we examined the skin of patients with type 2 diabetes and found that their microvascular perfusion was significantly compromised. This phenomenon was replicated in a high-fat diet-driven murine model of type 2 diabetes-like disease. In this setting, although both macrophages and mast cells were enriched in the skin, only mast cells and associated degranulation were critically required for the microvascular impairment. Sensory neurons exhibited enhanced TRPV1 activities, which triggered mast cells to degranulate and compromise skin microcirculation. Chemical and genetic ablation of TRPV1+ nociceptors robustly improved skin microcirculation status. Substance P (SP) is a neuropeptide and was elevated in the skin and sensory neurons in the context of type 2 diabetes. Exogenous administration of SP resulted in impaired skin microcirculation, whereas neuronal knockdown of SP dramatically prevented mast cell degranulation and consequently improved skin microcirculation. Overall, our findings indicate a neuron-mast cell axis underlying skin microcirculation disturbance in diabetes and shed light on neuroimmune therapeutics for diabetes-related complications.
© 2024 by the American Diabetes Association.
Conflict of interest statement
Figures


Comment in
-
The Sensory Neuron-Mast Cell Axis Regulation of Skin Microcirculation in Diabetes: Implication for Diabetes-Related Cutaneous Complications.Diabetes. 2024 Oct 1;73(10):1563-1565. doi: 10.2337/dbi24-0035. Diabetes. 2024. PMID: 39303086 Free PMC article. No abstract available.
References
-
- Faselis C, Katsimardou A, Imprialos K, Deligkaris P, Kallistratos M, Dimitriadis K. Microvascular complications of type 2 diabetes mellitus. Curr Vasc Pharmacol 2020;18:117–124 - PubMed
-
- Aubdool AA, Brain SD. Neurovascular aspects of skin neurogenic inflammation. J Invest Derm Symp Proc 2011;15:33–39 - PubMed
MeSH terms
Substances
Grants and funding
- 2022ZD0206200/Technological Innovation 2030-Major Projects of "Brain Science and Brain-like Research"
- 2022B1515020067/Guangdong Natural Science Funds for Distinguished Young Scholar
- 22ykqb03/Fundamental Research Funds for the Central Universities at Sun Yat-sen University
- yzjj2022rc02/President Foundation of ZhuJiang Hospital, Southern Medical University
- 81974192; 82271392; 82073427; 82273511/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

