Factors associated with immune responses to SARS-CoV-2 vaccination in individuals with autoimmune diseases
- PMID: 38833310
- PMCID: PMC11383356
- DOI: 10.1172/jci.insight.180750
Factors associated with immune responses to SARS-CoV-2 vaccination in individuals with autoimmune diseases
Abstract
Patients with autoimmune diseases are at higher risk for severe infection due to their underlying disease and immunosuppressive treatments. In this real-world observational study of 463 patients with autoimmune diseases, we examined risk factors for poor B and T cell responses to SARS-CoV-2 vaccination. We show a high frequency of inadequate anti-spike IgG responses to vaccination and boosting in the autoimmune population but minimal suppression of T cell responses. Low IgG responses in B cell-depleted patients with multiple sclerosis (MS) were associated with higher CD8 T cell responses. By contrast, patients taking mycophenolate mofetil (MMF) exhibited concordant suppression of B and T cell responses. Treatments with highest risk for low anti-spike IgG response included B cell depletion within the last year, fingolimod, and combination treatment with MMF and belimumab. Our data show that the mRNA-1273 (Moderna) vaccine is the most effective vaccine in the autoimmune population. There was minimal induction of either disease flares or autoantibodies by vaccination and no significant effect of preexisting anti-type I IFN antibodies on either vaccine response or breakthrough infections. The low frequency of breakthrough infections and lack of SARS-CoV-2-related deaths suggest that T cell immunity contributes to protection in autoimmune disease.
Keywords: Autoimmune diseases; Autoimmunity.
Figures
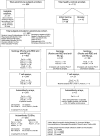

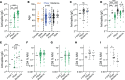
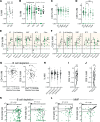
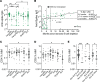

References
-
- Zecca E, et al. Ongoing mycophenolate treatment impairs anti-SARS-CoV-2 vaccination response in patients affected by chronic inflammatory autoimmune diseases or liver transplantation recipients: results of the RIVALSA prospective cohort. Viruses. 2022;14(8):1766. doi: 10.3390/v14081766. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

