CAR+ extracellular vesicles predict ICANS in patients with B cell lymphomas treated with CD19-directed CAR T cells
- PMID: 38833312
- PMCID: PMC11245152
- DOI: 10.1172/JCI173096
CAR+ extracellular vesicles predict ICANS in patients with B cell lymphomas treated with CD19-directed CAR T cells
Abstract
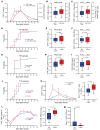
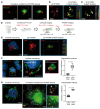
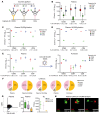
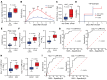
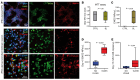
BACKGROUNDPredicting immune effector cell-associated neurotoxicity syndrome (ICANS) in patients infused with CAR T cells is still a conundrum. This complication, thought to be consequent to CAR T cell activation, arises a few days after infusion, when circulating CAR T cells are scarce and specific CAR T cell-derived biomarkers are lacking.METHODSCAR+ extracellular vesicle (CAR+EV) release was assessed in human CD19.CAR T cells cocultured with CD19+ target cells. A prospective cohort of 100 patients with B cell lymphoma infused with approved CD19.CAR T cell products was assessed for plasma CAR+EVs as biomarkers of in vivo CD19.CAR T cell activation. Human induced pluripotent stem cell-derived (iPSC-derived) neural cells were used as a model for CAR+EV-induced neurotoxicity.RESULTSIn vitro release of CAR+EVs occurs within 1 hour after target engagement. Plasma CAR+EVs are detectable 1 hour after infusion. A concentration greater than 132.8 CAR+EVs/μL at hour +1 or greater than 224.5 CAR+EVs/μL at day +1 predicted ICANS in advance of 4 days, with a sensitivity and a specificity outperforming other ICANS predictors. ENO2+ nanoparticles were released by iPSC-derived neural cells upon CAR+EV exposure and were increased in plasma of patients with ICANS.CONCLUSIONPlasma CAR+EVs are an immediate signal of CD19.CAR T cell activation, are suitable predictors of neurotoxicity, and may be involved in ICANS pathogenesis.TRIAL REGISTRATIONNCT04892433, NCT05807789.FUNDINGLife Science Hub-Advanced Therapies (financed by Health Ministry as part of the National Plan for Complementary Investments to the National Recovery and Resilience Plan [NRRP]: E.3 Innovative health ecosystem for APC fees and immunomonitoring).
Keywords: Hematology; Immunology; Immunotherapy; Lymphomas.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

