Multimodal imaging-based prediction of recurrence for unresectable HCC after downstage and resection-cohort study
- PMID: 38833331
- PMCID: PMC11392192
- DOI: 10.1097/JS9.0000000000001752
Multimodal imaging-based prediction of recurrence for unresectable HCC after downstage and resection-cohort study
Abstract
Background: Surgical resection (SR) following transarterial chemoembolization (TACE)-based downstaging is a promising treatment for unresectable hepatocellular carcinoma (uHCC), and identification of patients at high-risk of postoperative recurrence may assist individualized treatment.
Purpose: To develop and externally validate preoperative and postoperative prognostic models integrating multimodal CT and digital subtraction angiography features as well as clinico-therapeutic-pathological features for predicting disease-free survival (DFS) after TACE-based downstaging therapy.
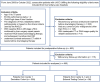
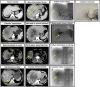
Materials and methods: From March 2008 to August 2022, 488 consecutive patients with Barcelona Clinic Liver Cancer (BCLC) A/B uHCC receiving TACE-based downstaging therapy and subsequent SR were included from four tertiary-care hospitals. All CT and digital subtraction angiography images were independently evaluated by two blinded radiologists. In the derivation cohort ( n =390), the XGBoost algorithm was used for feature selection, and Cox regression analysis for developing nomograms for DFS (time from downstaging to postoperative recurrence or death). In the external testing cohort ( n =98), model performances were compared with five major staging systems.
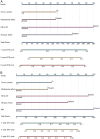
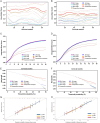
Results: The preoperative nomogram included over three tumors [hazard ratio (HR), 1.42; P =0.003], intratumoral artery (HR, 1.38; P =0.006), TACE combined with tyrosine kinase inhibitor (HR, 0.46; P <0.001) and objective response to downstaging therapy (HR, 1.60; P <0.001); while the postoperative nomogram included over three tumors (HR, 1.43; P =0.013), intratumoral artery (HR, 1.38; P =0.020), TACE combined with tyrosine kinase inhibitor (HR, 0.48; P <0.001), objective response to downstaging therapy (HR, 1.69; P <0.001) and microvascular invasion (HR, 2.20; P <0.001). The testing dataset C-indexes of the preoperative (0.651) and postoperative (0.687) nomograms were higher than all five staging systems (0.472-0.542; all P <0.001). Two prognostically distinct risk strata were identified according to these nomograms (all P <0.001).
Conclusion: Based on 488 patients receiving TACE-based downstaging therapy and subsequent SR for BCLC A/B uHCCs, the authors developed and externally validated two nomograms for predicting DFS, with superior performances than five major staging systems and effective survival stratification.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
All authors have no conflicts of interest to disclose.
Figures

Similar articles
-
Multiparametric MRI based deep learning model for prediction of early recurrence of hepatocellular carcinoma after SR following TACE.J Cancer Res Clin Oncol. 2024 Oct 8;150(10):448. doi: 10.1007/s00432-024-05941-w. J Cancer Res Clin Oncol. 2024. PMID: 39379692 Free PMC article.
-
Development of a Nomogram for Prognostic Prediction of Large Hepatocellular Carcinoma With HBV After TACE Combined Conversion Therapy.Hepat Med. 2025 Apr 7;17:1-12. doi: 10.2147/HMER.S481334. eCollection 2025. Hepat Med. 2025. PMID: 40226358 Free PMC article.
-
Multiparametric MRI-based radiomics and clinical nomogram predicts the recurrence of hepatocellular carcinoma after postoperative adjuvant transarterial chemoembolization.BMC Cancer. 2025 Apr 14;25(1):683. doi: 10.1186/s12885-025-14079-y. BMC Cancer. 2025. PMID: 40229712 Free PMC article.
-
Adjuvant transarterial chemoembolization improves survival outcomes in hepatocellular carcinoma with microvascular invasion: A systematic review and meta-analysis.Eur J Surg Oncol. 2019 Nov;45(11):2188-2196. doi: 10.1016/j.ejso.2019.06.031. Epub 2019 Jun 25. Eur J Surg Oncol. 2019. PMID: 31256949
-
Transarterial chemoembolization versus hepatic resection in hepatocellular carcinoma treatment: a meta-analysis.Drug Des Devel Ther. 2015 Aug 10;9:4431-40. doi: 10.2147/DDDT.S86629. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 26309396 Free PMC article. Review.
Cited by
-
A bibliometric and visual analysis based on immune checkpoint inhibitors for hepatocellular carcinoma: 2014 - 2024.Front Pharmacol. 2025 Apr 7;16:1520055. doi: 10.3389/fphar.2025.1520055. eCollection 2025. Front Pharmacol. 2025. PMID: 40260385 Free PMC article.
-
Machine learning model for predicting recurrence following intensity-modulated radiation therapy in nasopharyngeal carcinoma.World J Surg Oncol. 2025 Jun 18;23(1):237. doi: 10.1186/s12957-025-03860-9. World J Surg Oncol. 2025. PMID: 40533750 Free PMC article.
-
Diagnostic value of a lactylation-related gene signature in hepatocellular carcinoma.Transl Cancer Res. 2025 Jan 31;14(1):296-312. doi: 10.21037/tcr-24-1023. Epub 2025 Jan 23. Transl Cancer Res. 2025. PMID: 39974411 Free PMC article.
-
Noninvasive MRI imaging feature-based prediction of intratumoral tertiary lymphoid structure maturity in hepatocellular carcinoma: a multicenter retrospective study.Eur Radiol. 2025 Aug 6. doi: 10.1007/s00330-025-11902-9. Online ahead of print. Eur Radiol. 2025. PMID: 40767869
-
Development of a nomogram to predict risk factors for orchiectomy after testicular torsion in children.Sci Rep. 2025 Apr 30;15(1):15154. doi: 10.1038/s41598-025-97911-6. Sci Rep. 2025. PMID: 40307335 Free PMC article.
References
-
- Sung H, Ferlay J, Siegel RL, et al. . Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–249. - PubMed
-
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 2018;391:1301–1314. - PubMed
-
- European Association for the Study of the Liver . Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182–236. - PubMed
-
- Vitale A, Trevisani F, Farinati F, et al. . Treatment of hepatocellular carcinoma in the precision medicine era: from treatment stage migration to therapeutic hierarchy. Hepatology 2020;72:2206–2218. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

