Overcoming Resistance of Caco-2 Cells to 5-Fluorouracil through Diruthenium Complex Encapsulation in PMMA Nanoparticles
- PMID: 38833385
- PMCID: PMC11256753
- DOI: 10.1021/acs.inorgchem.4c01323
Overcoming Resistance of Caco-2 Cells to 5-Fluorouracil through Diruthenium Complex Encapsulation in PMMA Nanoparticles
Abstract
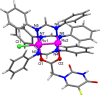
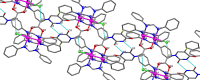
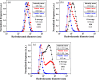
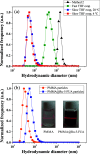
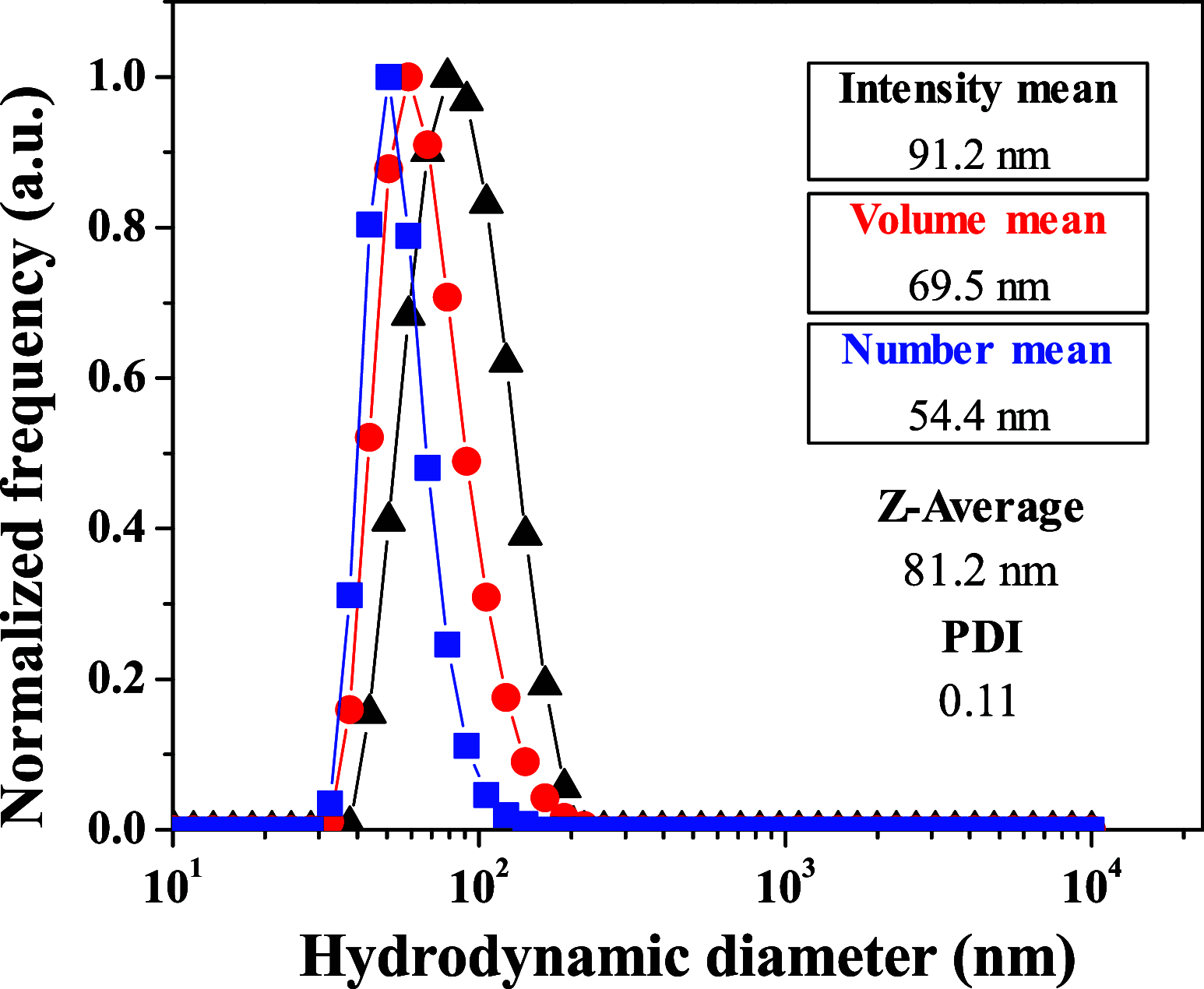
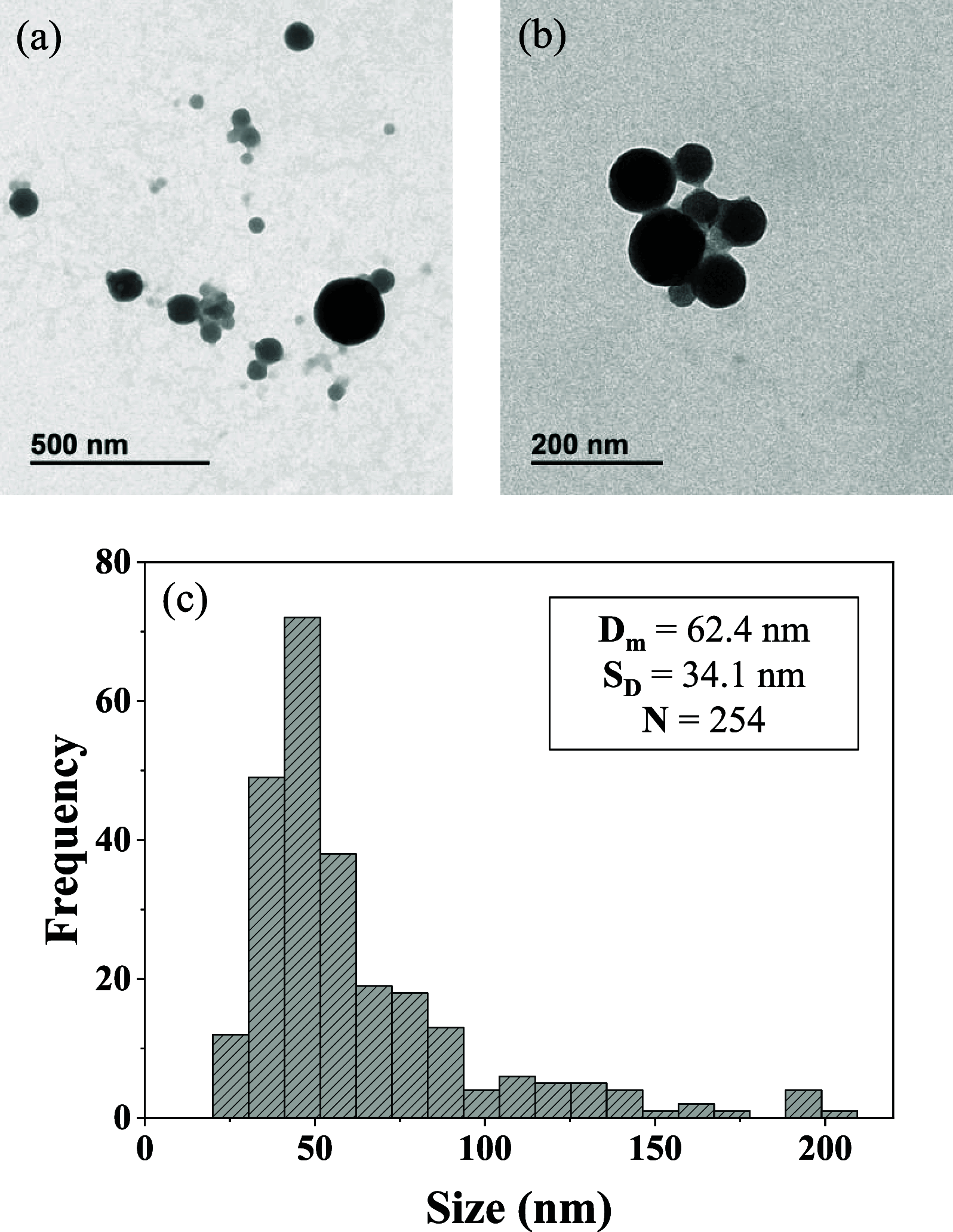
Drug resistance, one of the main drawbacks in cancer chemotherapy, can be tackled by employing a combination of drugs that target different biological processes in the cell, enhancing the therapeutic efficacy. Herein, we report the synthesis and characterization of a new paddlewheel diruthenium complex that includes 5-fluorouracil (5-FU), a commonly used anticancer drug. This drug was functionalized with a carboxylate group to take advantage of the previously demonstrated release capacity of carboxylate ligands from the diruthenium core. The resulting hydrophobic complex, [Ru2Cl(DPhF)3(5-FUA)] (Ru-5-FUA) (DPhF = N,N'-diphenylformamidinate; 5-FUA = 5-fluorouracil-1-acetate) was subsequently entrapped in poly(methyl methacrylate) (PMMA) nanoparticles (PMMA@Ru-5-FUA) via a reprecipitation method to be transported in biological media. The optimized encapsulation procedure yielded particles with an average size of 81.2 nm, a PDI of 0.11, and a zeta potential of 29.2 mV. The cytotoxicity of the particles was tested in vitro using the human colon carcinoma cell line Caco-2. The IC50 (half maximal inhibitory concentration) of PMMA@Ru-5-FUA (6.08 μM) was just slightly lower than that found for the drug 5-FU (7.64 μM). Most importantly, while cells seemed to have developed drug resistance against 5-FU, PMMA@Ru-5-FUA showed an almost complete lethality at ∼30 μM. Conversely, an analogous diruthenium complex devoid of the 5-FU moiety, [Ru2Cl(DPhF)3(O2CCH3)] (PMMA@RuA), displayed a reduced cytotoxicity at equivalent concentrations. These findings highlight the effect of combining the anticancer properties of 5-FU with those of diruthenium species. This suggests that the distinct modes of action of the two chemical species are crucial for overcoming drug resistance.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Hyphenation of lipophilic ruthenium(II)-diphosphine core with 5-fluorouracil: an effective metallodrug against glioblastoma brain cancer cells.Dalton Trans. 2024 Jan 23;53(4):1551-1567. doi: 10.1039/d3dt02941g. Dalton Trans. 2024. PMID: 38164612
-
Effect of N,N Coordination and RuII Halide Bond in Enhancing Selective Toxicity of a Tyramine-Based RuII (p-Cymene) Complex.Inorg Chem. 2020 May 4;59(9):6581-6594. doi: 10.1021/acs.inorgchem.0c00694. Epub 2020 Apr 15. Inorg Chem. 2020. PMID: 32295347
-
Comparative Analysis of the Inhibitory Mechanism of Aβ1-42 Aggregation by Diruthenium Complexes.Inorg Chem. 2024 May 27;63(21):10001-10010. doi: 10.1021/acs.inorgchem.4c01218. Epub 2024 May 14. Inorg Chem. 2024. PMID: 38742626
-
Detailed account on activation mechanisms of ruthenium coordination complexes and their role as antineoplastic agents.Eur J Med Chem. 2018 Apr 25;150:419-445. doi: 10.1016/j.ejmech.2018.03.015. Epub 2018 Mar 7. Eur J Med Chem. 2018. PMID: 29547831 Review.
-
Ruthenium Complexes as Anticancer Agents: A Brief History and Perspectives.Drug Des Devel Ther. 2020 Dec 3;14:5375-5392. doi: 10.2147/DDDT.S275007. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 33299303 Free PMC article. Review.
References
-
- Paprocka R.; Wiese-Szadkowska M.; Janciauskiene S.; Kosmalski T.; Kulik M.; Helmin-Basa A. Latest Developments in Metal Complexes as Anticancer Agents. Coord. Chem. Rev. 2022, 452, 21430710.1016/j.ccr.2021.214307. - DOI
-
- Calvert H. The Clinical Development of Carboplatin – A Personal Perspective. Inorg. Chim. Acta 2019, 498, 11898710.1016/j.ica.2019.118987. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

