Geospatial joint modeling of vector and parasite serology to microstratify malaria transmission
- PMID: 38833464
- PMCID: PMC11181033
- DOI: 10.1073/pnas.2320898121
Geospatial joint modeling of vector and parasite serology to microstratify malaria transmission
Abstract
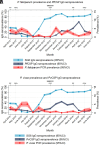
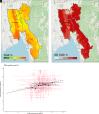
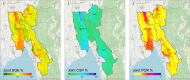
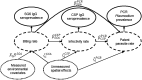
The World Health Organization identifies a strong surveillance system for malaria and its mosquito vector as an essential pillar of the malaria elimination agenda. Anopheles salivary antibodies are emerging biomarkers of exposure to mosquito bites that potentially overcome sensitivity and logistical constraints of traditional entomological surveys. Using samples collected by a village health volunteer network in 104 villages in Southeast Myanmar during routine surveillance, the present study employs a Bayesian geostatistical modeling framework, incorporating climatic and environmental variables together with Anopheles salivary antigen serology, to generate spatially continuous predictive maps of Anopheles biting exposure. Our maps quantify fine-scale spatial and temporal heterogeneity in Anopheles salivary antibody seroprevalence (ranging from 9 to 99%) that serves as a proxy of exposure to Anopheles bites and advances current static maps of only Anopheles occurrence. We also developed an innovative framework to perform surveillance of malaria transmission. By incorporating antibodies against the vector and the transmissible form of malaria (sporozoite) in a joint Bayesian geostatistical model, we predict several foci of ongoing transmission. In our study, we demonstrate that antibodies specific for Anopheles salivary and sporozoite antigens are a logistically feasible metric with which to quantify and characterize heterogeneity in exposure to vector bites and malaria transmission. These approaches could readily be scaled up into existing village health volunteer surveillance networks to identify foci of residual malaria transmission, which could be targeted with supplementary interventions to accelerate progress toward elimination.
Keywords: Anopheles salivary antibodies; disease mapping; geospatial; malaria.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures
Similar articles
-
Anopheles salivary antigens as serological biomarkers of vector exposure and malaria transmission: A systematic review with multilevel modelling.Elife. 2021 Dec 23;10:e73080. doi: 10.7554/eLife.73080. Elife. 2021. PMID: 34939933 Free PMC article.
-
Human antibodies against Anopheles salivary proteins: emerging biomarkers of mosquito and malaria exposure.Trends Parasitol. 2025 May;41(5):361-373. doi: 10.1016/j.pt.2025.03.009. Epub 2025 Apr 16. Trends Parasitol. 2025. PMID: 40246632 Review.
-
Spatial-temporal heterogeneity in malaria receptivity is best estimated by vector biting rates in areas nearing elimination.Parasit Vectors. 2018 Nov 27;11(1):606. doi: 10.1186/s13071-018-3201-1. Parasit Vectors. 2018. PMID: 30482239 Free PMC article.
-
Human exposure to Anopheles farauti bites in the Solomon Islands is not associated with IgG antibody response to the gSG6 salivary protein of Anopheles gambiae.Malar J. 2019 Oct 1;18(1):334. doi: 10.1186/s12936-019-2975-8. Malar J. 2019. PMID: 31570113 Free PMC article.
-
Assessment of Anopheles salivary antigens as individual exposure biomarkers to species-specific malaria vector bites.Malar J. 2012 Dec 31;11:439. doi: 10.1186/1475-2875-11-439. Malar J. 2012. PMID: 23276246 Free PMC article.
Cited by
-
Distribution of Anophelinae (Diptera: Culicidae) and challenges for malaria elimination in Brazil.Mem Inst Oswaldo Cruz. 2025 Feb 24;120:e240247. doi: 10.1590/0074-02760240247. eCollection 2025. Mem Inst Oswaldo Cruz. 2025. PMID: 40008702 Free PMC article.
-
Development of high affinity antibodies to Plasmodium falciparum merozoite and sporozoite antigens during infancy and adulthood.Front Immunol. 2025 Jul 2;16:1562671. doi: 10.3389/fimmu.2025.1562671. eCollection 2025. Front Immunol. 2025. PMID: 40672955 Free PMC article.
References
-
- World Health Organization, World Malaria Report 2021 (WHO, Geneva, 2021). https://www.who.int/publications/i/item/9789240040496. Accessed 8 June 2022.
-
- World Health Organization, Strategy for malaria elimination in the Greater Mekong Subregion: 2015–2030 (WHO, Geneva, 2015). https://www.who.int/publications/i/item/9789290617181. Accessed 8 June 2022.
MeSH terms
Grants and funding
- 1134989/DHAC | National Health and Medical Research Council (NHMRC)
- 2009656/DHAC | National Health and Medical Research Council (NHMRC)
- 2018654/DHAC | National Health and Medical Research Council (NHMRC)
- 1166753/DHAC | National Health and Medical Research Council (NHMRC)
- 1196068/DHAC | National Health and Medical Research Council (NHMRC)
LinkOut - more resources
Full Text Sources
Medical

