Cu(II) complex that synergistically potentiates cytotoxicity and an antitumor immune response by targeting cellular redox homeostasis
- PMID: 38833473
- PMCID: PMC11181140
- DOI: 10.1073/pnas.2404668121
Cu(II) complex that synergistically potentiates cytotoxicity and an antitumor immune response by targeting cellular redox homeostasis
Abstract
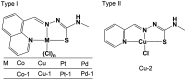
Developing anticancer drugs with low side effects is an ongoing challenge. Immunogenic cell death (ICD) has received extensive attention as a potential synergistic modality for cancer immunotherapy. However, only a limited set of drugs or treatment modalities can trigger an ICD response and none of them have cytotoxic selectivity. This provides an incentive to explore strategies that might provide more effective ICD inducers free of adverse side effects. Here, we report a metal-based complex (Cu-1) that disrupts cellular redox homeostasis and effectively stimulates an antitumor immune response with high cytotoxic specificity. Upon entering tumor cells, this Cu(II) complex enhances the production of intracellular radical oxidative species while concurrently depleting glutathione (GSH). As the result of heightening cellular oxidative stress, Cu-1 gives rise to a relatively high cytotoxicity to cancer cells, whereas normal cells with low levels of GSH are relatively unaffected. The present Cu(II) complex initiates a potent ferroptosis-dependent ICD response and effectively inhibits in vivo tumor growth in an animal model (c57BL/6 mice challenged with colorectal cancer). This study presents a strategy to develop metal-based drugs that could synergistically potentiate cytotoxic selectivity and promote apoptosis-independent ICD responses through perturbations in redox homeostasis.
Keywords: disrupting redox homeostasis; ferroptosis; immunogenic cell death; metal complex.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures



Similar articles
-
Ultrasound (US)-activated redox dyshomeostasis therapy reinforced by immunogenic cell death (ICD) through a mitochondrial targeting liposomal nanosystem.Theranostics. 2021 Sep 13;11(19):9470-9491. doi: 10.7150/thno.62984. eCollection 2021. Theranostics. 2021. PMID: 34646381 Free PMC article.
-
Discovery of Cu(II)-Dipyridophenazine Complex for Synergistic Cuproptosis/Chemodynamic Therapy via Disrupting the Tricarboxylic Acid (TCA) Cycle in Metastatic TNBC.Small. 2025 Jul;21(26):e2504554. doi: 10.1002/smll.202504554. Epub 2025 May 9. Small. 2025. PMID: 40348584
-
The copper (II) complex of salicylate phenanthroline induces immunogenic cell death of colorectal cancer cells through inducing endoplasmic reticulum stress.Int Immunopharmacol. 2024 May 10;132:111980. doi: 10.1016/j.intimp.2024.111980. Epub 2024 Mar 30. Int Immunopharmacol. 2024. PMID: 38555819
-
Anticancer activity of metal complexes: involvement of redox processes.Antioxid Redox Signal. 2011 Aug 15;15(4):1085-127. doi: 10.1089/ars.2010.3663. Epub 2011 May 11. Antioxid Redox Signal. 2011. PMID: 21275772 Free PMC article. Review.
-
Redox-implications associated with the formation of complexes between copper ions and reduced or oxidized glutathione.J Inorg Biochem. 2016 Jan;154:78-88. doi: 10.1016/j.jinorgbio.2015.08.005. Epub 2015 Aug 11. J Inorg Biochem. 2016. PMID: 26277412 Review.
Cited by
-
Metal-based immunogenic cell death inducers for cancer immunotherapy.Chem Sci. 2025 Feb 25;16(15):6160-6187. doi: 10.1039/d4sc08495k. eCollection 2025 Apr 9. Chem Sci. 2025. PMID: 40160356 Free PMC article. Review.
-
Tumor microenvironment and immune-related myositis: addressing muscle wasting in cancer immunotherapy.Front Immunol. 2025 May 2;16:1580108. doi: 10.3389/fimmu.2025.1580108. eCollection 2025. Front Immunol. 2025. PMID: 40386783 Free PMC article. Review.
-
Mechanism and application of copper-based nanomedicines in activating tumor immunity through oxidative stress modulation.Front Pharmacol. 2025 Jul 11;16:1646890. doi: 10.3389/fphar.2025.1646890. eCollection 2025. Front Pharmacol. 2025. PMID: 40717985 Free PMC article. Review.
-
Recent progress in stimuli-activable metallo-prodrugs for cancer therapy.Smart Mol. 2024 Aug 29;2(3):e20240030. doi: 10.1002/smo.20240030. eCollection 2024 Sep. Smart Mol. 2024. PMID: 40625908 Free PMC article. Review.
References
-
- Kroemer G., Galluzzi L., Kepp O., Zitvogel L., Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 31, 51–72 (2013). - PubMed
-
- Englinger B., et al. , Metal drugs and the anticancer immune response. Chem. Rev. 119, 1519–1624 (2018). - PubMed
-
- Galluzzi L., Buque A., Kepp O., Zitvogel L., Kroemer G., Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 17, 97–111 (2017). - PubMed
-
- Wong D. Y. Q., Ong W. W. F., Ang W. H., Induction of immunogenic cell death by chemotherapeutic platinum complexes. Angew. Chem. Int. Ed. Engl. 54, 6483–6487 (2015). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

