Maternal obesity increases hypothalamic miR-505-5p expression in mouse offspring leading to altered fatty acid sensing and increased intake of high-fat food
- PMID: 38833481
- PMCID: PMC11149872
- DOI: 10.1371/journal.pbio.3002641
Maternal obesity increases hypothalamic miR-505-5p expression in mouse offspring leading to altered fatty acid sensing and increased intake of high-fat food
Abstract
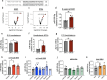
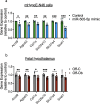
In utero exposure to maternal obesity programs increased obesity risk. Animal models show that programmed offspring obesity is preceded by hyperphagia, but the mechanisms that mediate these changes are unknown. Using a mouse model of maternal obesity, we observed increased intake of a high-fat diet (HFD) in offspring of obese mothers that precedes the development of obesity. Through small RNA sequencing, we identified programmed overexpression of hypothalamic miR-505-5p that is established in the fetus, lasts to adulthood and is maintained in hypothalamic neural progenitor cells cultured in vitro. Metabolic hormones and long-chain fatty acids associated with obesity increase miR-505-5p expression in hypothalamic neurons in vitro. We demonstrate that targets of miR-505-5p are enriched in fatty acid metabolism pathways and overexpression of miR-505-5p decreased neuronal fatty acid metabolism in vitro. miR-505-5p targets are associated with increased BMI in human genetic studies. Intra-cerebroventricular injection of miR-505-5p in wild-type mice increased HFD intake, mimicking the phenotype observed in offspring exposed to maternal obesity. Conversely, maternal exercise intervention in an obese mouse pregnancy rescued the programmed increase of hypothalamic miR-505-5p in offspring of obese dams and reduced HFD intake to control offspring levels. This study identifies a novel mechanism by which maternal obesity programs obesity in offspring via increased intake of high-fat foods.
Copyright: © 2024 Dearden et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

