A specific innate immune response silences the virulence of Pseudomonas aeruginosa in a latent infection model in the Drosophila melanogaster host
- PMID: 38833496
- PMCID: PMC11178223
- DOI: 10.1371/journal.ppat.1012252
A specific innate immune response silences the virulence of Pseudomonas aeruginosa in a latent infection model in the Drosophila melanogaster host
Abstract
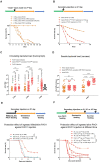
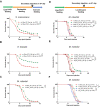
Microbial pathogenicity often depends on the route of infection. For instance, P. aeruginosa or S. marcescens cause acute systemic infections when low numbers of bacteria are injected into D. melanogaster flies whereas flies succumb much slower to the continuous ingestion of these pathogens, even though both manage to escape from the gut compartment and reach the hemocoel. Here, we have developed a latent P. aeruginosa infection model by feeding flies on the bacteria for a short period. The bacteria stably colonize internal tissues yet hardly cause any damage since latently-infected flies live almost as long as noninfected control flies. The apparently dormant bacteria display particular characteristics in terms of bacterial colony morphology, composition of the outer cell wall, and motility. The virulence of these bacteria can however be reactivated upon wounding the host. We show that melanization but not the cellular or the systemic humoral response is the predominant host defense that establishes latency and may coerce the bacteria to a dormant state. In addition, the lasting activation of the melanization responses in latently-infected flies provides a degree of protection to the host against a secondary fungal infection. Latent infection by an ingested pathogen protects against a variety of homologous or heterologous systemic secondary infectious challenges, a situation previously described for the endosymbiotic Wolbachia bacteria, a guard against viral infections.
Copyright: © 2024 Chen et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Pseudomonas aeruginosa RhlR is required to neutralize the cellular immune response in a Drosophila melanogaster oral infection model.Proc Natl Acad Sci U S A. 2011 Oct 18;108(42):17378-83. doi: 10.1073/pnas.1114907108. Epub 2011 Oct 10. Proc Natl Acad Sci U S A. 2011. PMID: 21987808 Free PMC article.
-
Assessing Pseudomonas virulence with a nonmammalian host: Drosophila melanogaster.Methods Mol Biol. 2014;1149:723-40. doi: 10.1007/978-1-4939-0473-0_56. Methods Mol Biol. 2014. PMID: 24818946
-
Wolbachia-mediated antibacterial protection and immune gene regulation in Drosophila.PLoS One. 2011;6(9):e25430. doi: 10.1371/journal.pone.0025430. Epub 2011 Sep 29. PLoS One. 2011. PMID: 21980455 Free PMC article.
-
Small flies to tackle big questions: assaying complex bacterial virulence mechanisms using Drosophila melanogaster.Cell Microbiol. 2014 Jun;16(6):824-33. doi: 10.1111/cmi.12292. Epub 2014 Apr 3. Cell Microbiol. 2014. PMID: 24628939 Review.
-
Modeling Host-Pathogen Interactions in C. elegans: Lessons Learned from Pseudomonas aeruginosa Infection.Int J Mol Sci. 2024 Jun 27;25(13):7034. doi: 10.3390/ijms25137034. Int J Mol Sci. 2024. PMID: 39000143 Free PMC article. Review.
Cited by
-
Nora virus proliferates in dividing intestinal stem cells and sensitizes flies to intestinal infection and oxidative stress.bioRxiv [Preprint]. 2025 Feb 4:2025.01.30.635658. doi: 10.1101/2025.01.30.635658. bioRxiv. 2025. PMID: 39975242 Free PMC article. Preprint.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

