Comprehensive Target Engagement by the EZH2 Inhibitor Tulmimetostat Allows for Targeting of ARID1A Mutant Cancers
- PMID: 38833522
- PMCID: PMC11292196
- DOI: 10.1158/0008-5472.CAN-24-0398
Comprehensive Target Engagement by the EZH2 Inhibitor Tulmimetostat Allows for Targeting of ARID1A Mutant Cancers
Abstract
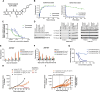
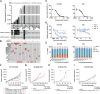
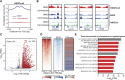
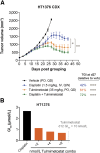
Recurrent somatic mutations in the BRG1/BRM-associated factor (BAF) chromatin remodeling complex subunit ARID1A occur frequently in advanced urothelial, endometrial, and ovarian clear cell carcinomas, creating an alternative chromatin state that may be exploited therapeutically. The histone methyltransferase EZH2 has been previously identified as targetable vulnerability in the context of ARID1A mutations. In this study, we describe the discovery of tulmimetostat, an orally available, clinical stage EZH2 inhibitor, and it elucidates the aspects of its application potential in ARID1A mutant tumors. Tulmimetostat administration achieved efficacy in multiple ARID1A mutant bladder, ovarian, and endometrial tumor models and improved cisplatin response in chemotherapy-resistant models. Consistent with its comprehensive and durable level of target coverage, tulmimetostat demonstrated greater efficacy than other PRC2-targeted inhibitors at comparable or lower exposures in a bladder cancer xenograft mouse model. Tulmimetostat mediated extensive changes in gene expression, in addition to a profound reduction in global H3K27me3 levels in tumors. Phase I clinical pharmacokinetic and pharmacodynamic data indicated that tulmimetostat exhibits durable exposure and profound target engagement. Importantly, a tulmimetostat controlled gene expression signature identified in whole blood from a cohort of 32 patients with cancer correlated with tulmimetostat exposure, representing a pharmacodynamic marker for the assessment of target coverage for PRC2-targeted agents in the clinic. Collectively, these data suggest that tulmimetostat has the potential to achieve clinical benefit in solid tumors as a monotherapy but also in combination with chemotherapeutic agents, and may be beneficial in various indications with recurrent ARID1A mutations. Significance: The EZH2 inhibitor tulmimetostat achieves comprehensive target inhibition in ARID1A mutant solid tumor models and cancer patients that can be assessed with a pharmacodynamic gene signature in peripheral blood.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
P.J. Keller reports a patent for 63/445,024 pending to Constellation Pharmaceuticals and was an employee of Constellation Pharmaceuticals, Inc., during time of work on publication. E.J. Adams reports a patent for WO2024015566(A1) pending; and E.J. Adams was an employee of Constellation Pharmaceuticals and MorphoSys US during time of work on publication. Third party funding of Constellation Pharmacueticals and MorphoSys AG can be found in their respective SEC filings. R. Meyer reports other support from Constellation Pharmaceuticals and MorphoSys during the conduct of the study. J.A. Mertz reports other support from Flare Therapeutics outside the submitted work. V. Gehling reports personal fees and other support from Odyssey Therapeutics and personal fees from Kura Oncology outside the submitted work; in addition, V. Gehling has a patent for WO/2019/204490 issued. J. Cui reports other support from Constellation Pharmaceuticals outside the submitted work and is an employee of Constellation Pharmaceuticals. J.I. Stuckey reports a patent for WO2019204490—Modulators of Methyl Modifying Enzymes, Compositions and Uses Thereof issued. A. Khanna reports a patent 20240116905 issued. Z. Yu reports a patent for EZH2 inhibition therapies for the treatment of AT-rich interactive domain-containing protein 1A (ARID1A) mutated cancers pending. N.J. Lakhani reports nonfinancial support and other support from MorphoSys/Constellation Pharma during the conduct of the study; other support from Arcus Biosciences, Artios, GSK, Celgene/BMS, Gilead, Ikena, InhibRx, Janssen, Jounce, KSQ/Roche, LOXO/Lilly, Macrogenics, Merck, Alkermes/Mural Oncology, Regeneron, Revolution Medicines, Repare Theraepeutics, Symphogen/Servier, Seagen/Pfizer, Shattuck Labs, Sapience, Tizona, SK Lifesciences, and Volastra; nonfinancial support and other support from Incyte. D. Rasco reports other support from MorphoSys during the conduct of the study and other support from 23 & Me, Arcus Biosciences, Adcentrx, Aulos, Bolt, BMS, Cullinan, Compugen USA, Inc., Flare, GSK, Eikon, JNJ, Kronos, AMAL, PureTech Health, Molecular Templates, and Takeda outside the submitted work. M. Gutierrez reports personal fees from Guardant Health, COTA Healthcare, Cellularity, Merck, BMS, Sanofi, and Incyte outside the submitted work; and research funding/institutional relationship with no financial interest: Acerta Pharma; Adlai Nortye; Arcus Biosciences; Array BioPharma; Bayer; Bellicum Pharmaceuticals; BMS; Boehringer Ingelheim; Celgene; Checkpoint Therapeutics; Compass Therapeutics; Constellation Pharmaceuticals; Cullinan Oncology; Cyteir; Daiichi Sankyo Company; Eisai; EMD Serono; EMD Serono; Erasca, Inc.; Fate Therapeutics; Georgetown University; GSK; GSB Pharma; Hackensack Meridian Health; Imugene; Incyte; Infinity Pharmaceuticals; ITeos Therapeutics; Janssen; Johnson & Johnson; KSQ Therapeutics; MedImmune; Memorial Sloan-Kettering Cancer Center; Merck; Millennium; Mirati Therapeutics; Moderna Therapeutics; NextCure; NextCure; Nimbus Therapeutics; Pfizer; Pharmacyclics; Rapa Therapeutics; Regeneron; Roche/Genentech; Sanofi; Seagen; Silenseed; Synlogic; Tesaro; Turning Point Therapeutics; Vedanta Biosciences; VelosBio; Verastem; and Vincerx Pharma. L. Duska reports grants from MorphoSys during the conduct of the study and personal fees from Aadi Bioscience and Regeneron outside the submitted work. J. Wang reports other support from Constellation Pharmaceutical outside the submitted work. K. Sun reports a patent for 63/455,024 pending. P. Trojer reports personal fees from Constellation Pharmaceuticals, Inc., and MorphoSys during the conduct of the study and personal fees from MorphoSys, Inc. and Fulcrum Therapeutics outside the submitted work; in addition, P. Trojer has a patent for WO2024015566(A1) pending. No disclosures were reported by the other authors.
Figures


References
-
- Conery AR, Rocnik JL, Trojer P. Small molecule targeting of chromatin writers in cancer. Nat Chem Biol 2022;18:124–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

