Metal-Ligand Cooperation with Thiols as Transient Cooperative Ligands: Acceleration and Inhibition Effects in (De)Hydrogenation Reactions
- PMID: 38833580
- PMCID: PMC11191399
- DOI: 10.1021/acs.accounts.4c00198
Metal-Ligand Cooperation with Thiols as Transient Cooperative Ligands: Acceleration and Inhibition Effects in (De)Hydrogenation Reactions
Abstract
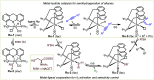
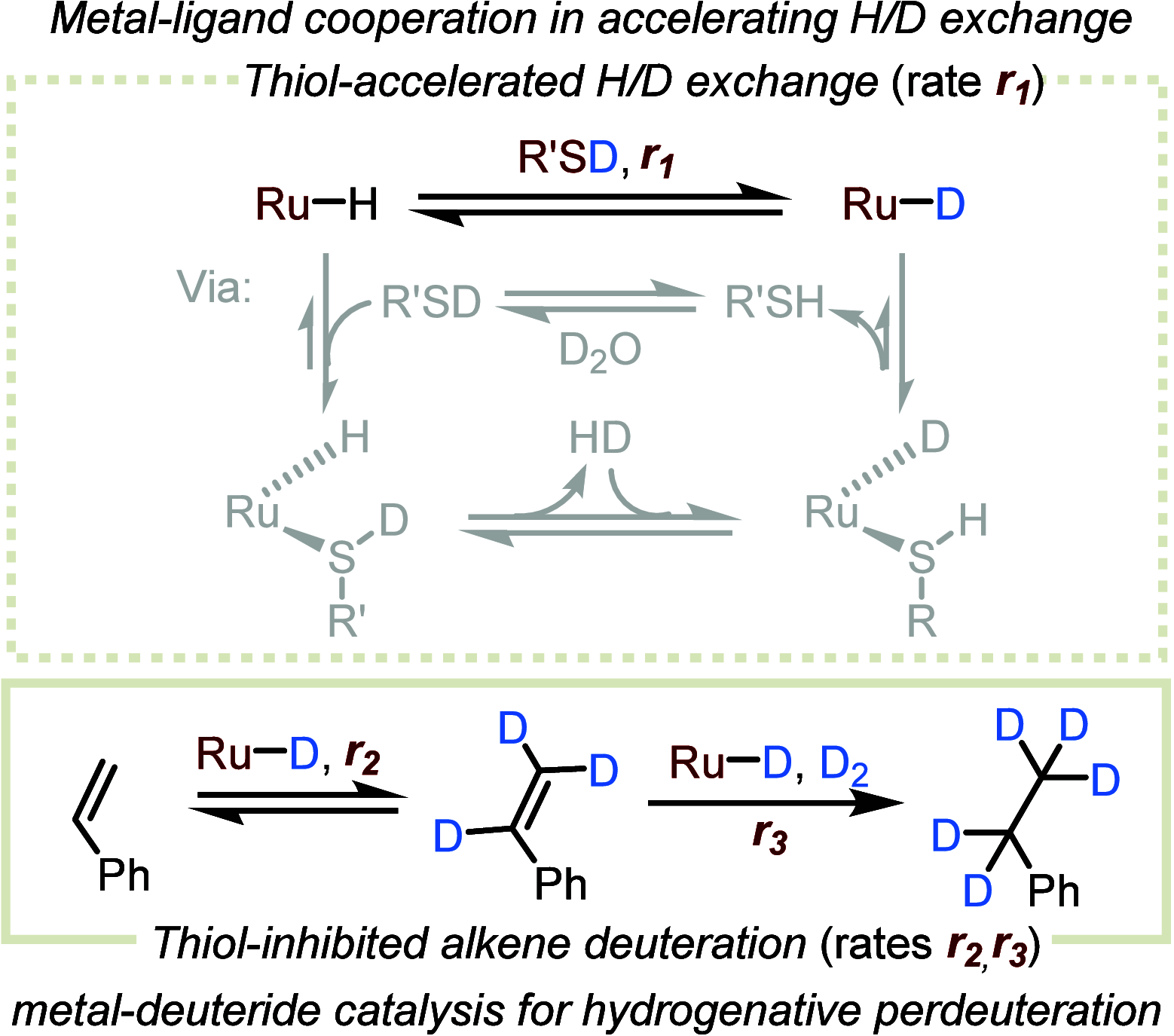
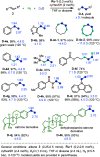
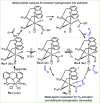
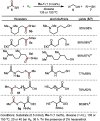
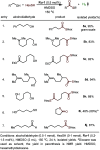
ConspectusOver the past two decades, we have developed a series of pincer-type transition metal complexes capable of activating strong covalent bonds through a mode of reactivity known as metal-ligand cooperation (MLC). In such systems, an incoming substrate molecule simultaneously interacts with both the metal center and ligand backbone, with one part of the molecule reacting at the metal center and another part at the ligand. The majority of these complexes feature pincer ligands with a pyridine core, and undergo MLC through reversible dearomatization/aromatization of this pyridine moiety. This MLC platform has enabled us to perform a variety of catalytic dehydrogenation, hydrogenation, and related reactions, with high efficiency and selectivity under relatively mild conditions.In a typical catalytic complex that operates through MLC, the cooperative ligand remains coordinated to the metal center throughout the entire catalytic process, and this complex is the only catalytic species involved in the reaction. As part of our ongoing efforts to develop new catalytic systems featuring MLC, we have recently introduced the concept of transient cooperative ligand (TCL), i.e., a ligand that is capable of MLC when coordinated to a metal center, but the coordination of which is reversible rather than permanent. We have thus far employed thiol(ate)s as TCLs, in conjunction with an acridanide-based ruthenium(II)-pincer catalyst, and this has resulted in remarkable acceleration and inhibition effects in various hydrogenation and dehydrogenation reactions. A cooperative thiol(ate) ligand can be installed in situ by the simple addition of an appropriate thiol in an amount equivalent to the catalyst, and this has been repeatedly shown to enable efficient bond activation by MLC without the need for other additives, such as base. The use of an ancillary thiol ligand that is not fixed to the pincer backbone allows the catalytic system to benefit from a high degree of tunability, easily implemented by varying the added thiol. Importantly, thiols are coordinatively labile enough under typical catalytic conditions to leave a meaningful portion of the catalyst in its original unsaturated form, thereby allowing it to carry out its own characteristic catalytic activity. This generates two coexisting catalyst populations─one that contains a thiol(ate) ligand and another that does not─and this may lead to different catalytic outcomes, namely, enhancement of the original catalytic activity, inhibition of this activity, or the occurrence of diverging reactivities within the same catalytic reaction mixture. These thiol effects have enabled us to achieve a series of unique transformations, such as thiol-accelerated base-free aqueous methanol reforming, controlled stereodivergent semihydrogenation of alkynes using thiol as a reversible catalyst inhibitor, and hydrogenative perdeuteration of C═C bonds without using D2, enabled by a combination of thiol-induced acceleration and inhibition. We have also successfully realized the unprecedented formation of thioesters through dehydrogenative coupling of alcohols and thiols, as well as the hydrogenation of organosulfur compounds, wherein the cooperative thiol serves as a reactant or product. In this Account, we present an overview of the TCL concept and its various applications using thiols.
Conflict of interest statement
The authors declare no competing financial interest.
Figures










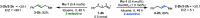






Similar articles
-
Hydrogenation and dehydrogenation iron pincer catalysts capable of metal-ligand cooperation by aromatization/dearomatization.Acc Chem Res. 2015 Jul 21;48(7):1979-94. doi: 10.1021/acs.accounts.5b00027. Epub 2015 Jun 16. Acc Chem Res. 2015. PMID: 26079678
-
Metal-ligand cooperation by aromatization-dearomatization: a new paradigm in bond activation and "green" catalysis.Acc Chem Res. 2011 Aug 16;44(8):588-602. doi: 10.1021/ar2000265. Epub 2011 Jul 8. Acc Chem Res. 2011. PMID: 21739968
-
A New Paradigm in Pincer Iridium Chemistry: PCN Complexes for (De)Hydrogenation Catalysis and Beyond.Acc Chem Res. 2022 Aug 2;55(15):2148-2161. doi: 10.1021/acs.accounts.2c00311. Epub 2022 Jul 19. Acc Chem Res. 2022. PMID: 35852837
-
Manganese Alkyl Carbonyl Complexes: From Iconic Stoichiometric Textbook Reactions to Catalytic Applications.Acc Chem Res. 2022 Sep 20;55(18):2740-2751. doi: 10.1021/acs.accounts.2c00470. Epub 2022 Sep 8. Acc Chem Res. 2022. PMID: 36074912 Free PMC article. Review.
-
Recent advances in osmium-catalyzed hydrogenation and dehydrogenation reactions.Acc Chem Res. 2015 Feb 17;48(2):363-79. doi: 10.1021/ar5003818. Epub 2015 Feb 4. Acc Chem Res. 2015. PMID: 25650714 Review.
Cited by
-
Introducing metal-sulfur active sites in metal-organic frameworks via post-synthetic modification for hydrogenation catalysis.Nat Chem. 2025 Jul 24. doi: 10.1038/s41557-025-01876-y. Online ahead of print. Nat Chem. 2025. PMID: 40707779
-
Long-Short-Arm Acridine Ru-Pincer Catalysts for Reversible Hydrogen Storage Based on Ethylene Glycol.J Am Chem Soc. 2025 Aug 20;147(33):30060-30071. doi: 10.1021/jacs.5c07428. Epub 2025 Aug 7. J Am Chem Soc. 2025. PMID: 40772821 Free PMC article.
-
Turning the Tables: Ligand-Centered Hydride Shuttling in Organometallic BIP-Al Systems.Inorg Chem. 2025 Aug 4;64(30):15760-15773. doi: 10.1021/acs.inorgchem.5c02587. Epub 2025 Jul 24. Inorg Chem. 2025. PMID: 40703003 Free PMC article.
References
-
- Luo J.; Rauch M.; Avram L.; Diskin-Posner Y.; Shmul G.; Ben-David Y.; Milstein D. Formation of Thioesters by Dehydrogenative Coupling of Thiols and Alcohols with H2 Evolution. Nat. Catal. 2020, 3, 887–892. 10.1038/s41929-020-00514-9. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

