Clinical characteristic and survival outcomes of patients with advanced NSCLC according to KRAS mutational status in the French real-life ESME cohort
- PMID: 38833966
- PMCID: PMC11179088
- DOI: 10.1016/j.esmoop.2024.103473
Clinical characteristic and survival outcomes of patients with advanced NSCLC according to KRAS mutational status in the French real-life ESME cohort
Abstract
Purpose: The RAS/MEK signaling pathway is essential in carcinogenesis and frequently altered in non-small-cell lung cancer (NSCLC), notably by KRAS mutations (KRASm) that affect 25%-30% of non-squamous NSCLC. This study aims to explore the impact of KRASm subtypes on disease phenotype and survival outcomes.
Patients and methods: We conducted a retrospective analysis of the French Epidemiological Strategy and Medical Economics database for advanced or metastatic lung cancer from 2011 to 2021. Patient demographics, histology, KRASm status, treatment strategies, and outcomes were assessed.
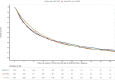
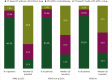
Results: Of 10 177 assessable patients for KRAS status, 17.6% had KRAS p.G12C mutation, 22.6% had KRAS non-p.G12C mutation, and 59.8% were KRASwt. KRASm patients were more often smokers (96.3%) compared with KRASwt (85.8%). A higher proportion of programmed death-ligand 1 ≥50% was found for KRASm patients: 43.5% versus 38.0% (P < 0.01). KRASm correlated with poorer outcomes. First-line median progression-free survival was shorter in the KRASm than the KRASwt cohort: 4.0 months [95% confidence interval (CI) 3.7-4.3 months] versus 5.1 months (95% CI 4.8-5.3 months), P < 0.001. First-line overall survival was shorter for KRASm than KRASwt patients: 12.6 months (95% CI 11.6-13.6 months) versus 15.4 months (95% CI 14.6-16.2 months), P = 0.012. First-line chemoimmunotherapy offered better overall survival in KRAS p.G12C (48.8 months) compared with KRAS non-p.G12C (24.0 months) and KRASwt (22.5 months) patients. Second-line overall survival with immunotherapy was superior in the KRAS p.G12C subgroup: 12.6 months (95% CI 8.1-18.6 months) compared with 9.4 months (95% CI 8.0-11.4 months) for KRAS non-p.G12C and 9.6 months (8.4-11.0 months) for KRASwt patients.
Conclusion: We highlighted distinct clinical profiles and survival outcomes according to KRASm subtypes. Notably KRAS p.G12C mutations may provide increased sensitivity to immunotherapy, suggesting potential therapeutic implications for sequencing or combination of therapies. Further research on the impact of emerging KRAS specific inhibitors are warranted in real-world cohorts.
Keywords: KRAS mutational status; NSCLC; immunotherapy; prognosis; real life data.
Copyright © 2024 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Disclosure QDT received honoraria from Amgen, AstraZeneca, and Sanofi and meeting/travel support from Amgen and Sanofi. CC received grants or contracts, consulting fees, personal/institutional honoraria, and meeting/travel support from AstraZeneca, Amgen, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, Lilly, Merck Sharp & Dohme (MSD), Novartis, Pfizer, Roche, Sanofi, and Takeda. TF received personal/institutional honoraria from Janssen, Lilly, and Roche. JRM received grants or contracts from MSD, received payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing or educational events from AstraZeneca, Edimark, Janssen, MSD, Sanofi, Roche, and Takeda and meeting/travel support from Ose-Immunotherapeutics. MP received consulting fees from AstraZeneca, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly, Eisai, GlaxoSmithKline, Ipsen, Janssen, MSD, Novocure, Pfizer, Roche, and Takeda; payment or honoraria for lectures, presentations, speaker bureau, manuscript writing or educational events from Anheart Therapeutics, AstraZeneca, Bristol-Myers Squibb, Janssen, MSD, Pfizer, Sanofi, and Takeda; payment for expert testimony from AstraZeneca, Bristol-Myers Squibb, Janssen, and Roche; support for attending meetings and/or travel from AstraZeneca, Bristol-Myers Squibb, MSD, Pfizer, Roche, and Takeda; and participated on a data safety monitoring board or advisory board for Pharmamar and Roche. CAV received consulting fees or meeting/travel support from Amgen, AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Lilly, MSD, Novartis, Pfizer, Roche, Sanofi Aventis, and Takeda; and participated in an advisory board for AbbVie, AstraZeneca, Bristol-Myers Squibb, Janssen, Lilly, MSD, Pfizer, Roche, and Sanofi. GJ received meeting/travel support from Sanofi. SH received personal/institutional honoraria from AstraZeneca, Bristol-Myers Squibb, Roche, Sanofi, and Takeda; and meeting/travel support from Novartis and Sanofi. AS received consulting fees from Exafield and Guidepoint; honoraria from Amgen, AstraZeneca, and MSD; meeting/travel support from Amgen and Roche; and is a board member of the SFFPO. EP received personal/institutional honoraria from Amgen, AstraZeneca, and MSD; meeting/travel support from Takeda and Amgen; and participated in an advisory board for Takeda. NG declared research grants/support from AbbVie, Amgen, AstraZeneca, BeiGene, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Gilead, Hoffmann-La Roche, Janssen, LEO Pharma, Lilly, Merk Serono, MSD, Novartis, Sanofi, Sivan; consultative services for AbbVie, Amgen, AstraZeneca, BeiGene, Bristol-Myers Squibb, Daiichi-Sankyo, Gilead, Ipsen Hoffmann-La Roche, Janssen, LEO Pharma, Lilly, MSD, Mirati, Novartis, Pfizer, Pierre Fabre, Sanofi, Takeda; participation on a data safety monitoring board for Hoffmann-La Roche; and employment of a family member with AstraZeneca. PDR received meeting/travel support from Boehringer Ingelheim, Daiichi Sankyo, Summit Therapeutics, Sanofi, and Takeda; and participated in an advisory board for Sanofi and Takeda. All other authors have declared no conflicts of interest.
Figures


Similar articles
-
Outcomes in patients treated with frontline immune checkpoint inhibition (ICI) for advanced NSCLC with KRAS mutations and STK11/KEAP1 comutations across PD-L1 levels.Lung Cancer. 2024 Apr;190:107510. doi: 10.1016/j.lungcan.2024.107510. Epub 2024 Feb 24. Lung Cancer. 2024. PMID: 38432028 Free PMC article.
-
Decoding KRAS mutation in non-small cell lung cancer patients receiving immunotherapy: A retrospective institutional comparison and literature review.Lung Cancer. 2025 Jan;199:108051. doi: 10.1016/j.lungcan.2024.108051. Epub 2024 Dec 9. Lung Cancer. 2025. PMID: 39740426 Review.
-
Outcome of First-Line Treatment With Pembrolizumab According to KRAS/TP53 Mutational Status for Nonsquamous Programmed Death-Ligand 1-High (≥50%) NSCLC in the German National Network Genomic Medicine Lung Cancer.J Thorac Oncol. 2024 May;19(5):803-817. doi: 10.1016/j.jtho.2023.12.015. Epub 2023 Dec 13. J Thorac Oncol. 2024. PMID: 38096950
-
Brief Report: Not Created Equal: Survival Differences by KRAS Mutation Subtype in NSCLC Treated With Immunotherapy.JTO Clin Res Rep. 2024 Oct 24;6(1):100755. doi: 10.1016/j.jtocrr.2024.100755. eCollection 2025 Jan. JTO Clin Res Rep. 2024. PMID: 39758602 Free PMC article.
-
Prevalence of KRAS G12C Mutation and Co-mutations and Associated Clinical Outcomes in Patients With Colorectal Cancer: A Systematic Literature Review.Oncologist. 2023 Nov 2;28(11):e981-e994. doi: 10.1093/oncolo/oyad138. Oncologist. 2023. PMID: 37432264 Free PMC article.
Cited by
-
Optimizing Treatment Strategies for Egfr-Mutated Non-Small-Cell Lung Cancer Treated with Osimertinib: Real-World Outcomes and Insights.Cancers (Basel). 2024 Oct 23;16(21):3563. doi: 10.3390/cancers16213563. Cancers (Basel). 2024. PMID: 39518004 Free PMC article.
-
Trends in Overall Survival in Lung Adenocarcinoma with EFGR Mutation, KRAS Mutation, or No Mutation.Cancers (Basel). 2025 Apr 5;17(7):1237. doi: 10.3390/cancers17071237. Cancers (Basel). 2025. PMID: 40227775 Free PMC article.
References
-
- Yang S.R., Schultheis A.M., Yu H., Mandelker D., Ladanyi M., Büttner R. Precision medicine in non-small cell lung cancer: current applications and future directions. Semin Cancer Biol. 2022;84:184–198. - PubMed
-
- Yates L.R., Seoane J., Le Tourneau C., et al. The European Society for Medical Oncology (ESMO) precision medicine glossary. Ann Oncol. 2018;29(1):30–35. - PubMed
-
- Drosten M., Barbacid M. Targeting the MAPK pathway in KRAS-driven tumors. Cancer Cell. 2020;37(4):543–550. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

