Cryo-EM structures of pathogenic fibrils and their impact on neurodegenerative disease research
- PMID: 38834068
- PMCID: PMC11257806
- DOI: 10.1016/j.neuron.2024.05.012
Cryo-EM structures of pathogenic fibrils and their impact on neurodegenerative disease research
Abstract
Neurodegenerative diseases are commonly associated with the formation of aberrant protein aggregates within the brain, and ultrastructural analyses have revealed that the proteins within these inclusions often assemble into amyloid filaments. Cryoelectron microscopy (cryo-EM) has emerged as an effective method for determining the near-atomic structure of these disease-associated filamentous proteins, and the resulting structures have revolutionized the way we think about aberrant protein aggregation and propagation during disease progression. These structures have also revealed that individual fibril conformations may dictate different disease conditions, and this newfound knowledge has improved disease modeling in the lab and advanced the ongoing pursuit of clinical tools capable of distinguishing and targeting different pathogenic entities within living patients. In this review, we summarize some of the recently developed cryo-EM structures of ex vivo α-synuclein, tau, β-amyloid (Aβ), TAR DNA-binding protein 43 (TDP-43), and transmembrane protein 106B (TMEM106B) fibrils and discuss how these structures are being leveraged toward mechanistic research and therapeutic development.
Keywords: Aβ; TDP-43; TMEM106B; amyloid; cryo-EM; tau; α-synuclein.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests L.P. is a consultant for Expansion Therapeutics.
Figures
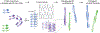
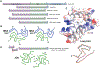



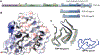
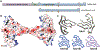
References
-
- Virchow R. (1854). Ueber eine im Gehirn und Rückenmark des Menschen aufgefundene Substanz mit der chemischen Reaction der Cellulose. Archiv für pathologische Anatomie und Physiologie und für klinische Medicin 6, 135–138. 10.1007/BF01930815. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

