Protein Haptenation and Its Role in Allergy
- PMID: 38834188
- PMCID: PMC11187640
- DOI: 10.1021/acs.chemrestox.4c00062
Protein Haptenation and Its Role in Allergy
Abstract
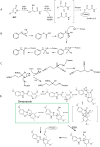
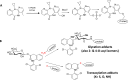
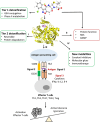
Humans are exposed to numerous electrophilic chemicals either as medicines, in the workplace, in nature, or through use of many common cosmetic and household products. Covalent modification of human proteins by such chemicals, or protein haptenation, is a common occurrence in cells and may result in generation of antigenic species, leading to development of hypersensitivity reactions. Ranging in severity of symptoms from local cutaneous reactions and rhinitis to potentially life-threatening anaphylaxis and severe hypersensitivity reactions such as Stephen-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), all these reactions have the same Molecular Initiating Event (MIE), i.e. haptenation. However, not all individuals who are exposed to electrophilic chemicals develop symptoms of hypersensitivity. In the present review, we examine common chemistry behind the haptenation reactions leading to formation of neoantigens. We explore simple reactions involving single molecule additions to a nucleophilic side chain of proteins and complex reactions involving multiple electrophilic centers on a single molecule or involving more than one electrophilic molecule as well as the generation of reactive molecules from the interaction with cellular detoxification mechanisms. Besides generation of antigenic species and enabling activation of the immune system, we explore additional events which result directly from the presence of electrophilic chemicals in cells, including activation of key defense mechanisms and immediate consequences of those reactions, and explore their potential effects. We discuss the factors that work in concert with haptenation leading to the development of hypersensitivity reactions and those that may act to prevent it from developing. We also review the potential harnessing of the specificity of haptenation in the design of potent covalent therapeutic inhibitors.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Stable isotope labeling method for the investigation of protein haptenation by electrophilic skin sensitizers.Toxicol Sci. 2014 Nov;142(1):239-49. doi: 10.1093/toxsci/kfu168. Epub 2014 Aug 21. Toxicol Sci. 2014. PMID: 25145658
-
Haptenation of sulfonamide reactive metabolites to cellular proteins.Mol Pharmacol. 2002 Nov;62(5):1011-26. doi: 10.1124/mol.62.5.1011. Mol Pharmacol. 2002. PMID: 12391263
-
Protein haptenation by amoxicillin: high resolution mass spectrometry analysis and identification of target proteins in serum.J Proteomics. 2012 Dec 21;77:504-20. doi: 10.1016/j.jprot.2012.09.030. Epub 2012 Oct 4. J Proteomics. 2012. PMID: 23041134
-
Current Perspectives on Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis.Clin Rev Allergy Immunol. 2018 Feb;54(1):147-176. doi: 10.1007/s12016-017-8654-z. Clin Rev Allergy Immunol. 2018. PMID: 29188475 Review.
-
[Anaphylactic reactions to low-molecular weight chemicals].Postepy Hig Med Dosw (Online). 2015 Feb 6;69:197-206. Postepy Hig Med Dosw (Online). 2015. PMID: 25661919 Review. Polish.
Cited by
-
Prediction of Respiratory Irritation and Respiratory Sensitization of Chemicals Using Structural Alerts and Machine Learning Modeling.Toxics. 2025 Mar 25;13(4):243. doi: 10.3390/toxics13040243. Toxics. 2025. PMID: 40278559 Free PMC article.
-
Terpene Hydroperoxides as Lipid Peroxidation Inducers: Biomimetic and HaCaT Cell Studies in Allergic Contact Dermatitis.Contact Dermatitis. 2025 Jul;93(1):16-30. doi: 10.1111/cod.14804. Epub 2025 May 1. Contact Dermatitis. 2025. PMID: 40312052 Free PMC article.
-
Limitations and Modifications of Skin Sensitization NAMs for Testing Inorganic Nanomaterials.Toxics. 2024 Aug 21;12(8):616. doi: 10.3390/toxics12080616. Toxics. 2024. PMID: 39195718 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

