Downregulation of rhodopsin is an effective therapeutic strategy in ameliorating peripherin-2-associated inherited retinal disorders
- PMID: 38834544
- PMCID: PMC11150396
- DOI: 10.1038/s41467-024-48846-5
Downregulation of rhodopsin is an effective therapeutic strategy in ameliorating peripherin-2-associated inherited retinal disorders
Erratum in
-
Author Correction: Downregulation of rhodopsin is an effective therapeutic strategy in ameliorating peripherin-2-associated inherited retinal disorders.Nat Commun. 2024 Jul 19;15(1):6091. doi: 10.1038/s41467-024-50140-3. Nat Commun. 2024. PMID: 39030161 Free PMC article. No abstract available.
Abstract
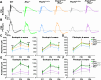
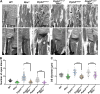
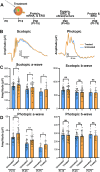
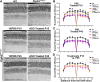
Given the absence of approved treatments for pathogenic variants in Peripherin-2 (PRPH2), it is imperative to identify a universally effective therapeutic target for PRPH2 pathogenic variants. To test the hypothesis that formation of the elongated discs in presence of PRPH2 pathogenic variants is due to the presence of the full complement of rhodopsin in absence of the required amounts of functional PRPH2. Here we demonstrate the therapeutic potential of reducing rhodopsin levels in ameliorating disease phenotype in knockin models for p.Lys154del (c.458-460del) and p.Tyr141Cys (c.422 A > G) in PRPH2. Reducing rhodopsin levels improves physiological function, mitigates the severity of disc abnormalities, and decreases retinal gliosis. Additionally, intravitreal injections of a rhodopsin-specific antisense oligonucleotide successfully enhance the physiological function of photoreceptors and improves the ultrastructure of discs in mutant mice. Presented findings shows that reducing rhodopsin levels is an effective therapeutic strategy for the treatment of inherited retinal degeneration associated with PRPH2 pathogenic variants.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
- P30 EY005722/EY/NEI NIH HHS/United States
- EY005722/U.S. Department of Health & Human Services | NIH | National Eye Institute (NEI)
- R01 EY030451/EY/NEI NIH HHS/United States
- EY033763/U.S. Department of Health & Human Services | NIH | National Eye Institute (NEI)
- EY030451/U.S. Department of Health & Human Services | NIH | National Eye Institute (NEI)
- R01 EY034671/EY/NEI NIH HHS/United States
- R56 EY010609/EY/NEI NIH HHS/United States
- K99 EY033763/EY/NEI NIH HHS/United States
- EY034671/U.S. Department of Health & Human Services | NIH | National Eye Institute (NEI)
- EY010609/U.S. Department of Health & Human Services | NIH | National Eye Institute (NEI)
- R01 EY010609/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

